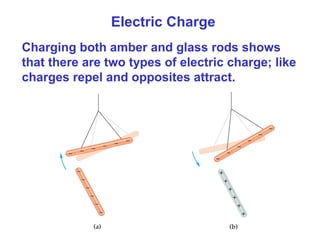
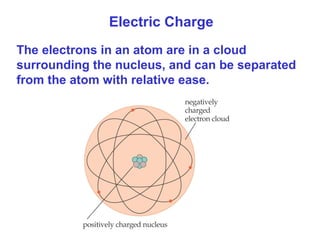
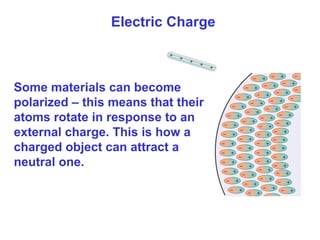
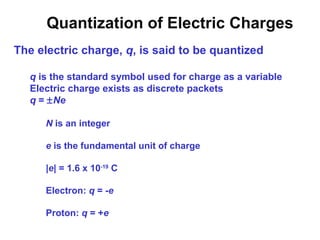
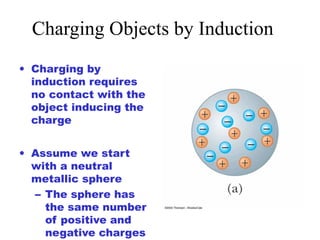
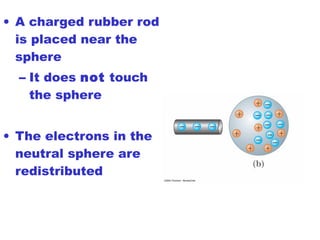
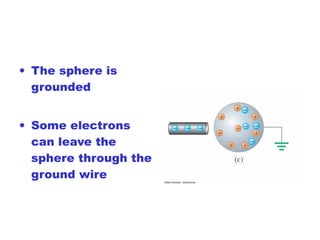
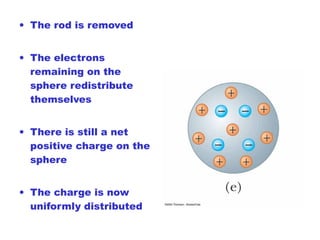
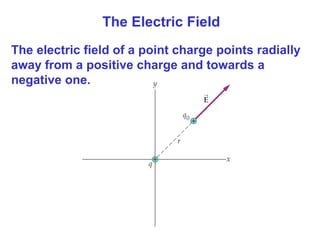
- Electric charge exists in two types, positive and negative, and is quantized in units of the elementary charge e. Charges of the same type repel, while opposite charges attract.
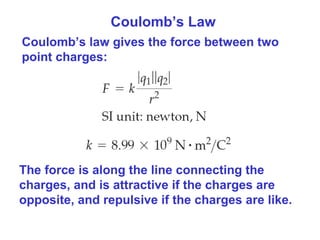
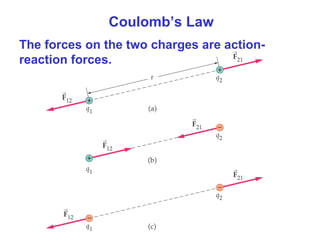
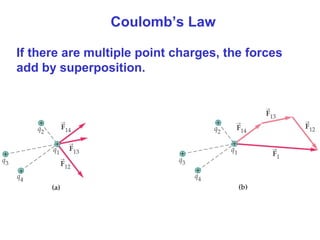
- Coulomb's law describes the electrostatic force between two point charges, directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
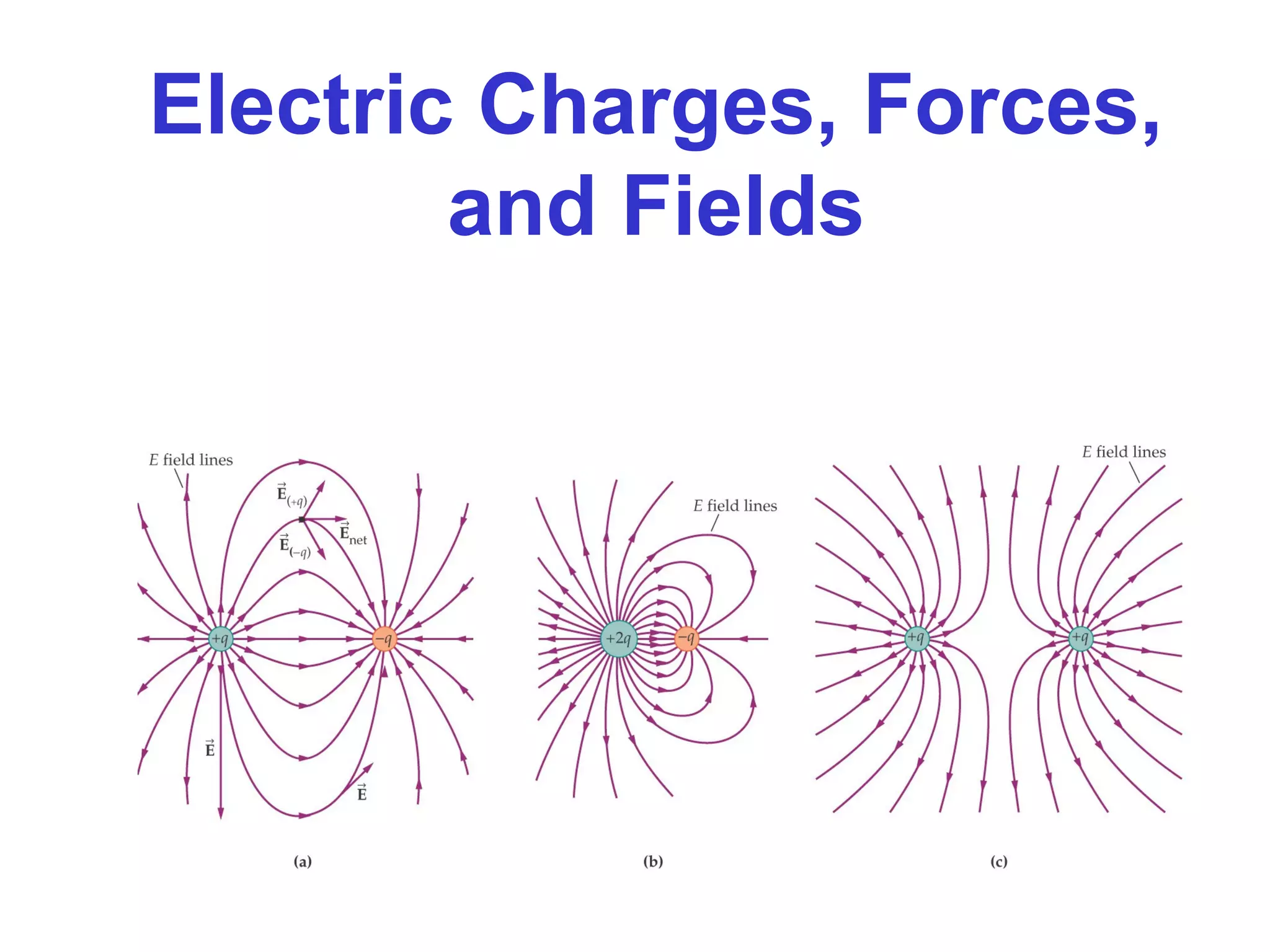
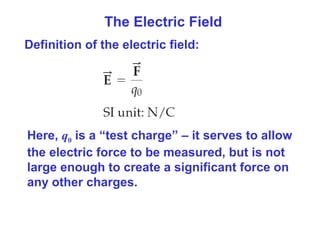
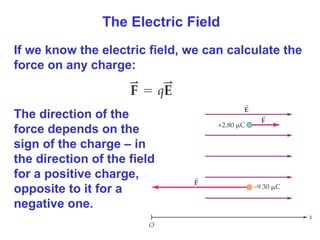
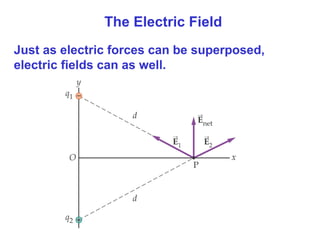
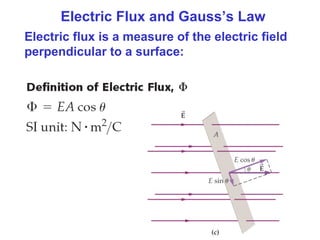
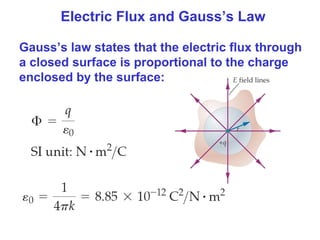
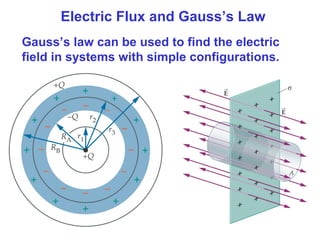
- The electric field is defined as the force per unit charge exerted on a test charge at some point, and can be used to determine the force on any charge. Electric fields from multiple sources add through superposition.