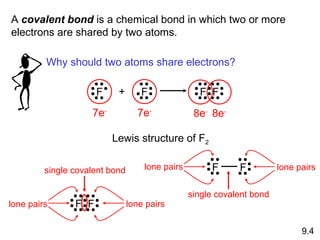
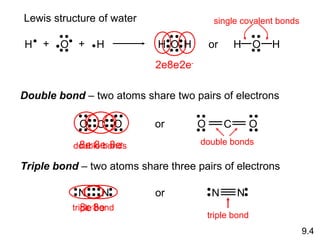
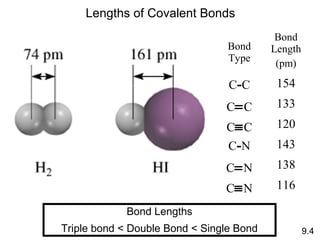
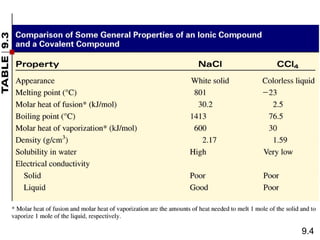
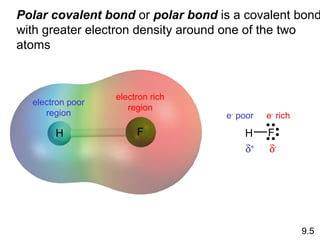
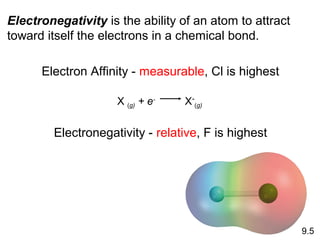
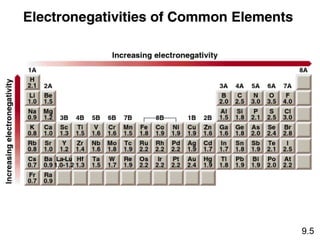
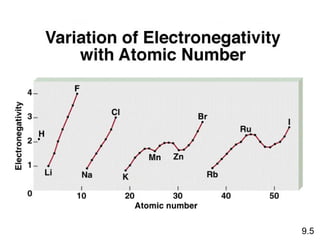
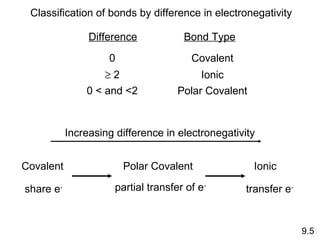
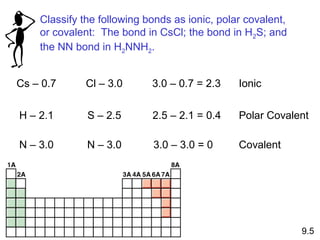
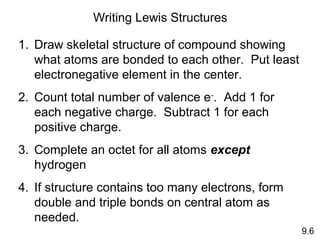
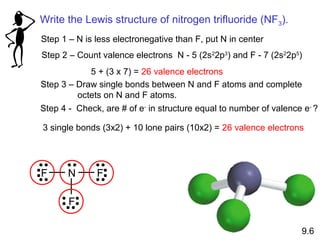
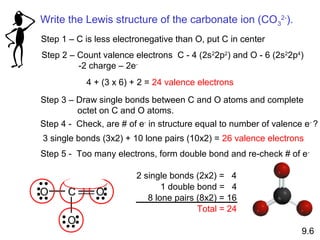
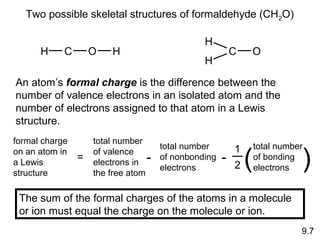
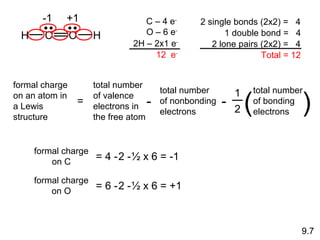
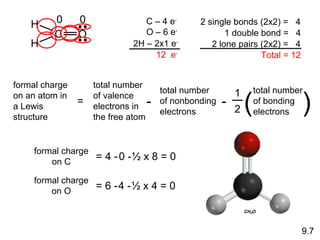
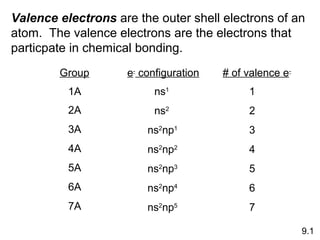
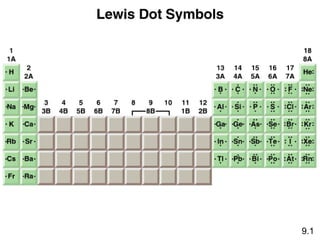
This document discusses chemical bonding and Lewis structures. It begins by defining valence electrons and explaining how they participate in bonding. The document then covers ionic bonding, covalent bonding including single, double and triple bonds, and polar covalent bonding. It introduces electronegativity and provides rules for classifying bonds. The remainder of the document focuses on writing Lewis structures, including counting electrons, placing bonds and lone pairs, and calculating formal charges.
![The Ionic Bond
Li + F Li+ F -
1s22s1 22s22p5
1s [He]2Ne]2p6
1s2[ 2s2
1s
Li Li+ + e-
-
e + F F -
Li+ + F - Li+ F -
9.2](https://image.slidesharecdn.com/chemicalbonding-basicconcepts-130402105236-phpapp02/85/Chemical-Bonding-Basic-Concepts-4-320.jpg)