Embed presentation
Downloaded 68 times











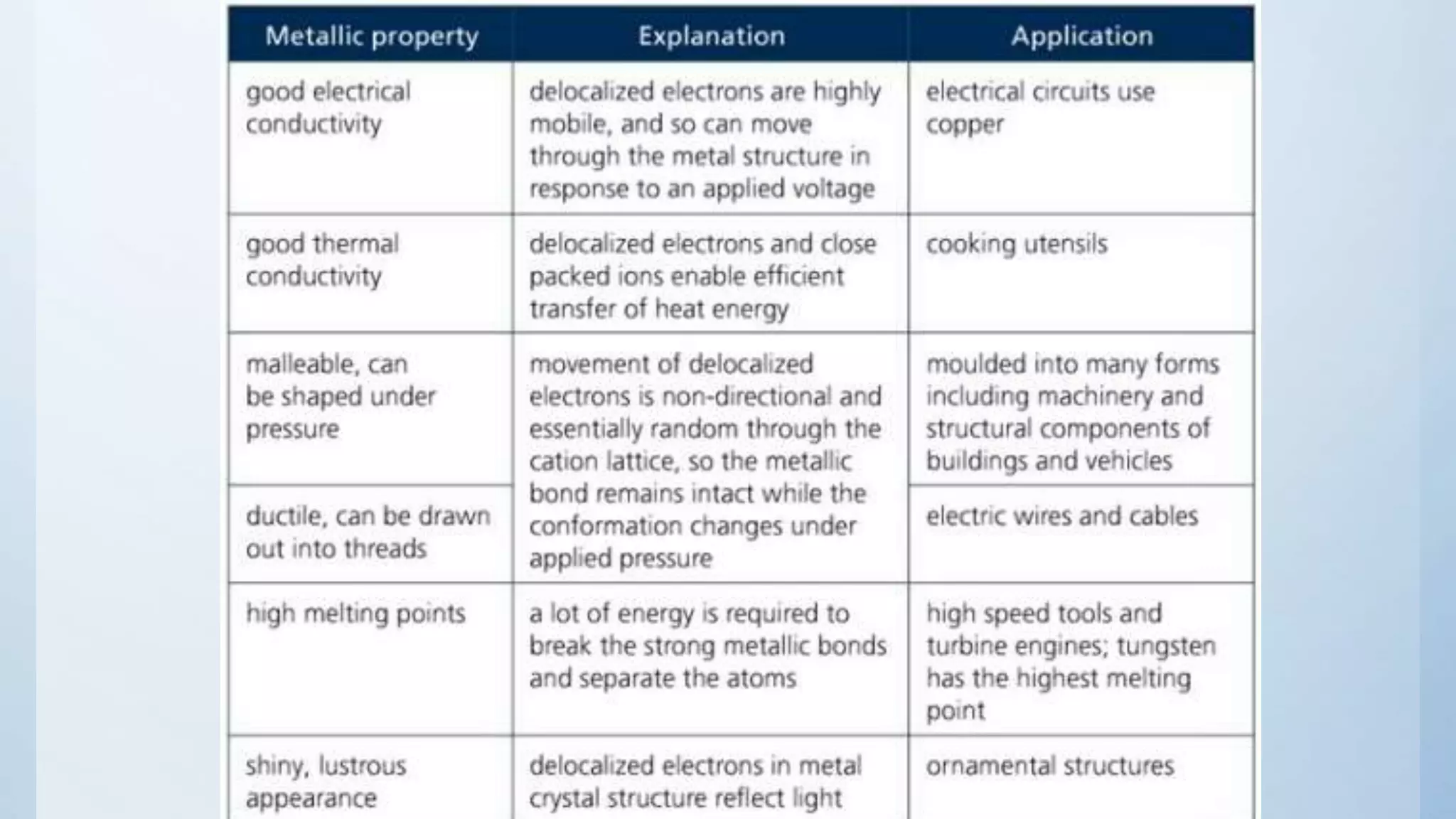

Metals form giant metallic structures through metallic bonding where the valence electrons are delocalized across the entire structure and are shared between all the metal atoms. This delocalization of electrons allows the metal atoms to move and shift positions while still maintaining the metallic bond through the "sea" of delocalized electrons. Metallic bonding explains the malleability and ductility of metals as the atoms can easily shift positions within the giant metallic structure.











