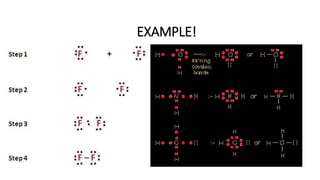
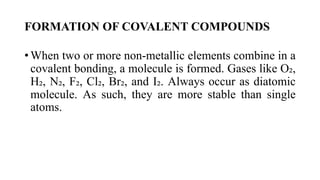
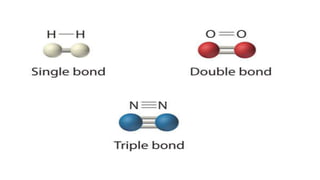
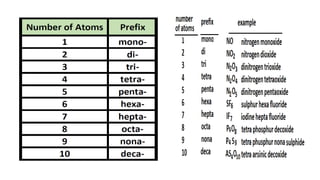
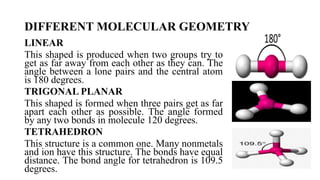
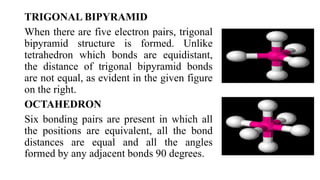
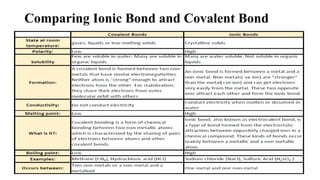
This document provides an overview of chemical bonding, including ionic and covalent bonds. It explains that ionic bonds form when ions transfer electrons, while covalent bonds form when atoms share electrons. The octet rule and Lewis electron dot diagrams are introduced to show how atoms gain or share electrons to achieve stable electron configurations like noble gases. Ionic compounds are formed from metals transferring electrons to nonmetals, resulting in cations and anions that bond ionically. Covalent compounds are formed by nonmetals sharing electrons in molecules. Molecular geometry is also discussed, including the shapes of molecules based on the number of electron pairs around the central atom.