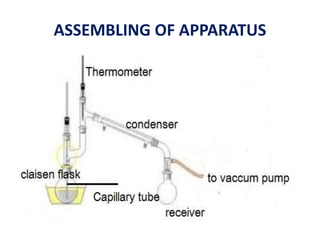
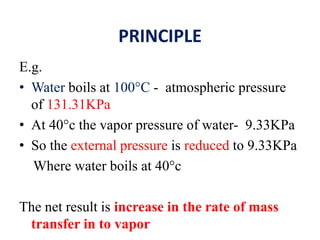
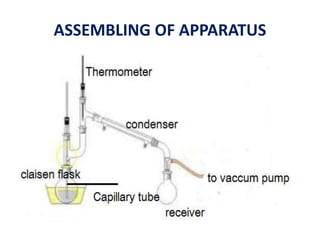
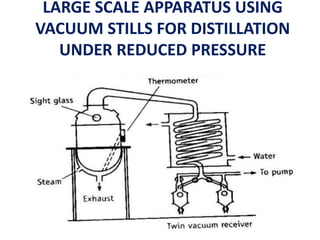
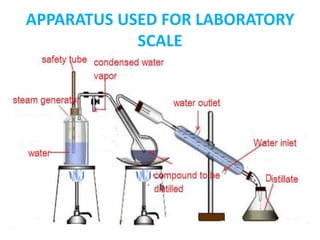
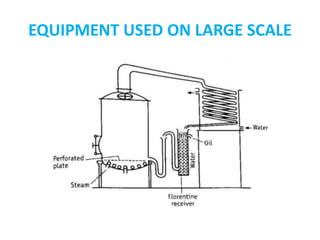
This document discusses distillation under reduced pressure and steam distillation. It describes the principles, construction of apparatus, procedures, advantages and disadvantages. Distillation under reduced pressure involves boiling a liquid at a lower temperature by reducing external pressure. Steam distillation allows separation of immiscible liquids or purification of high boiling substances by using steam. The key components and working of laboratory and large scale equipment are explained.