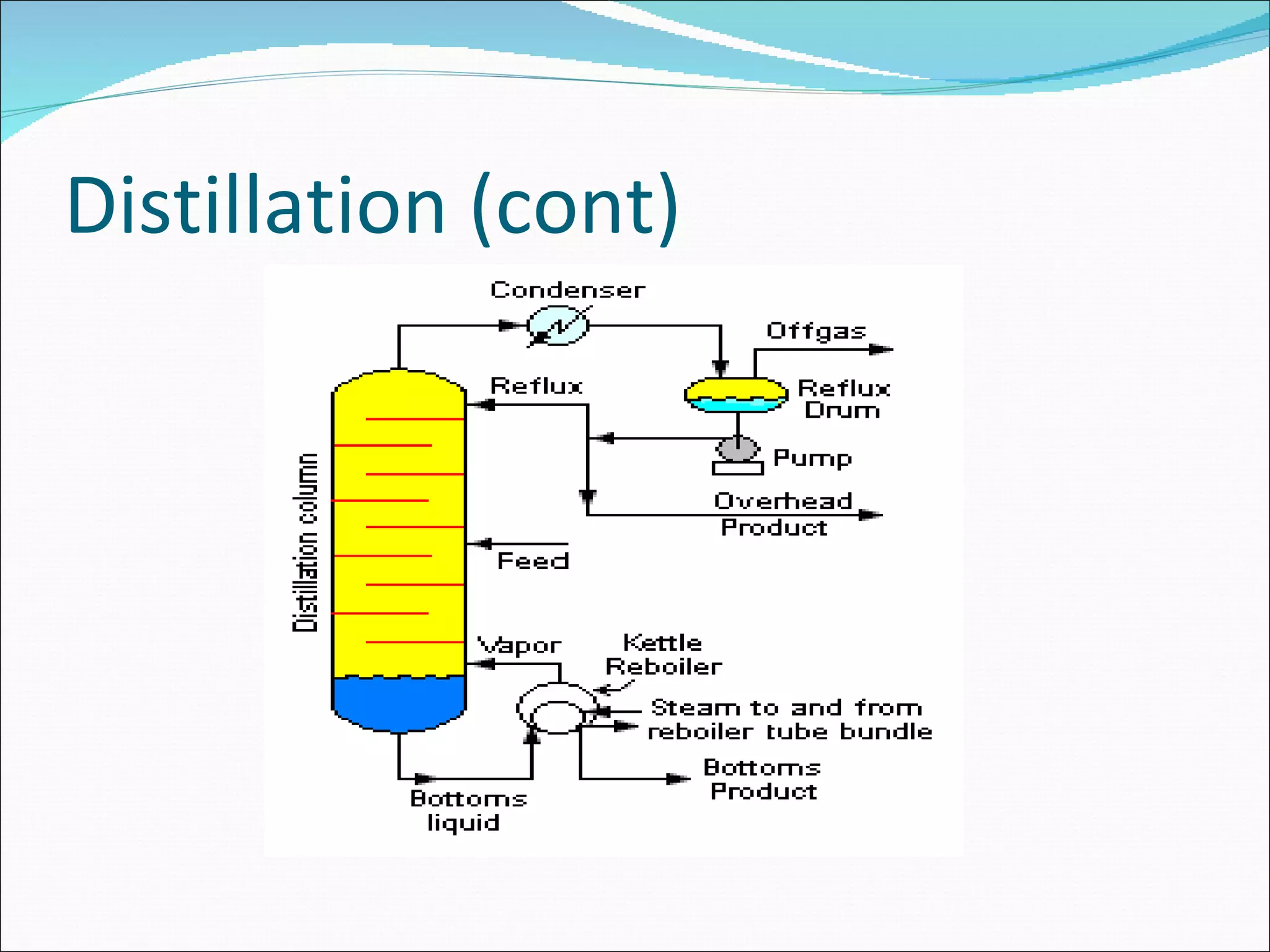
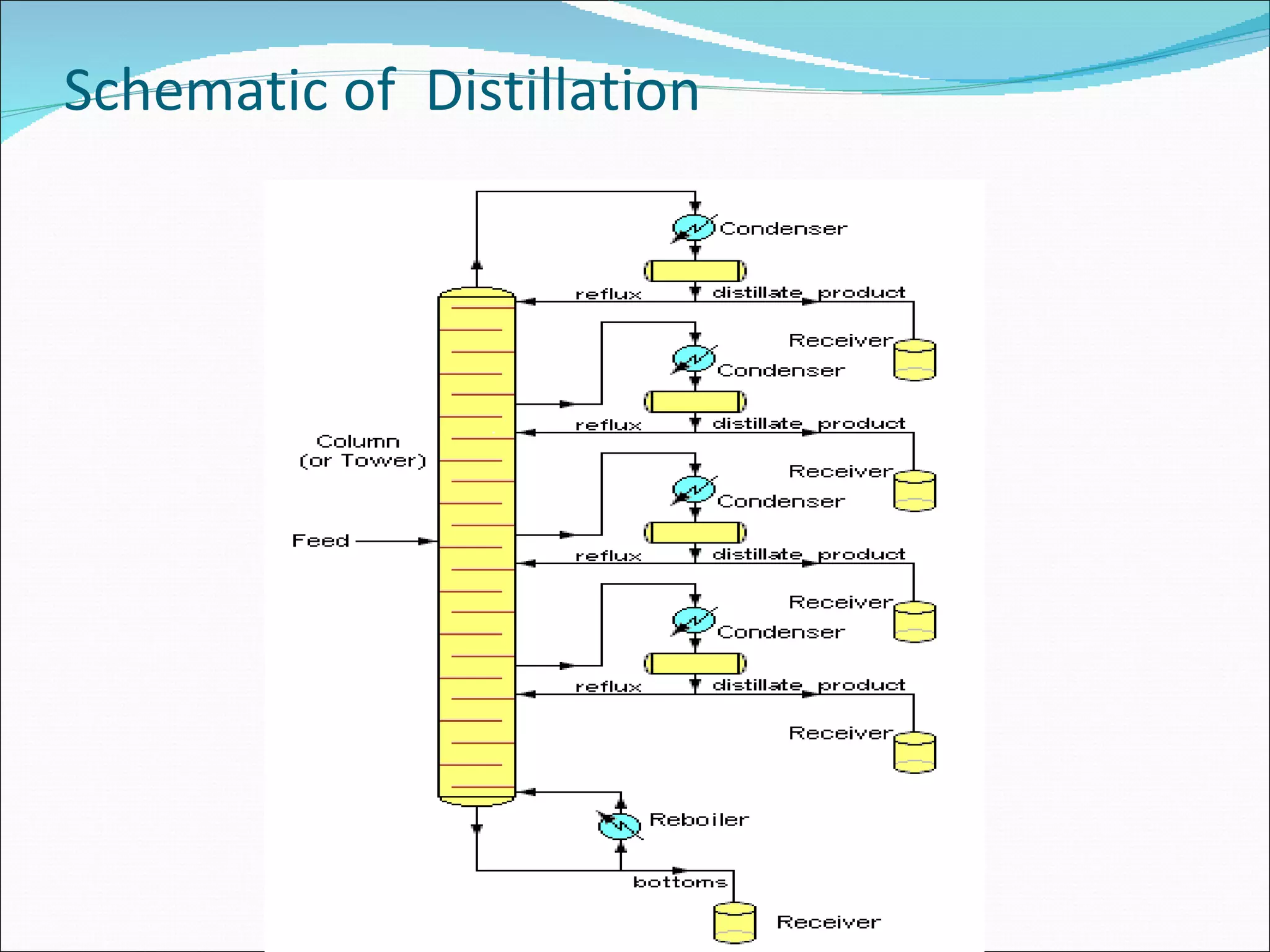
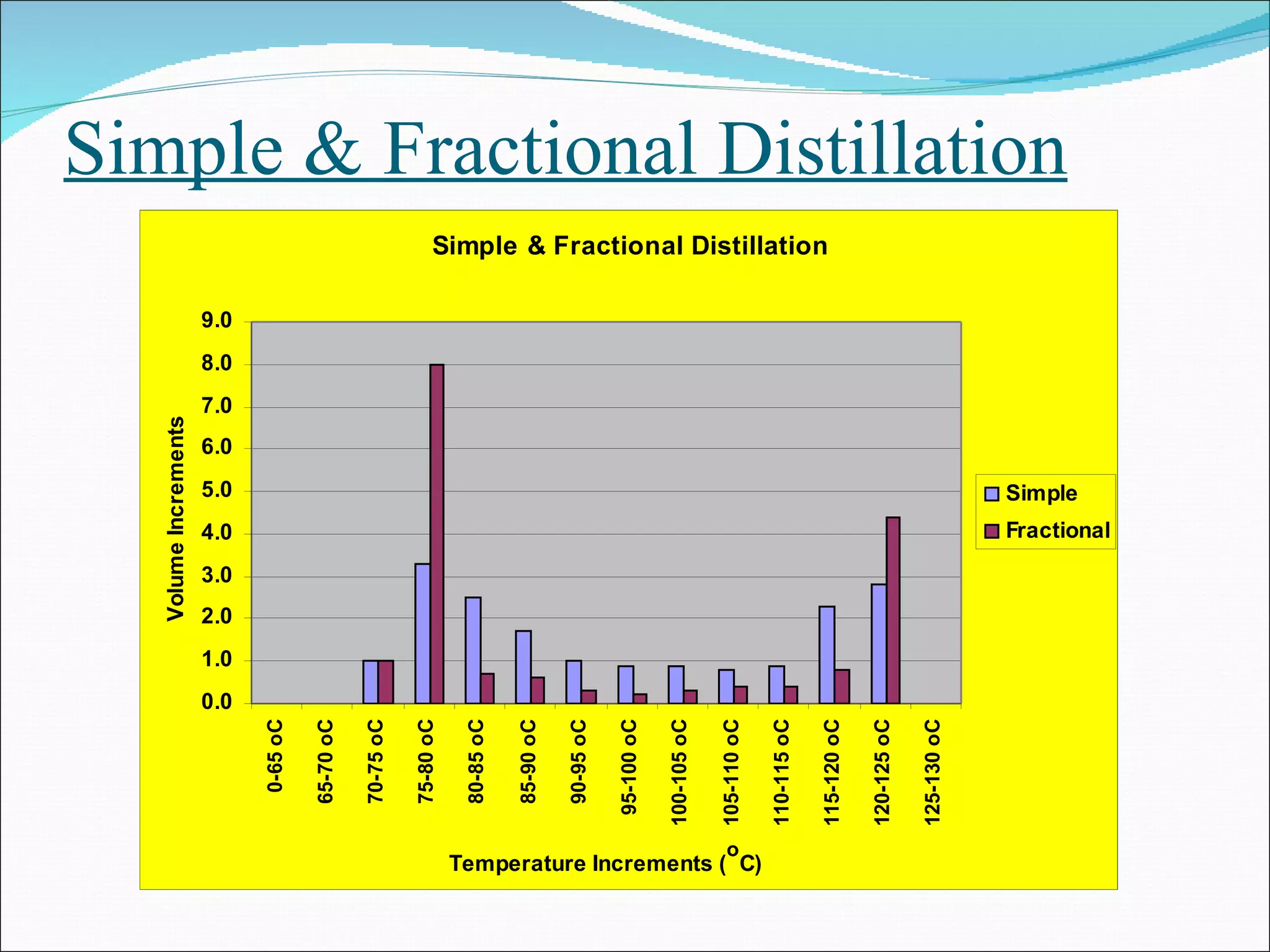
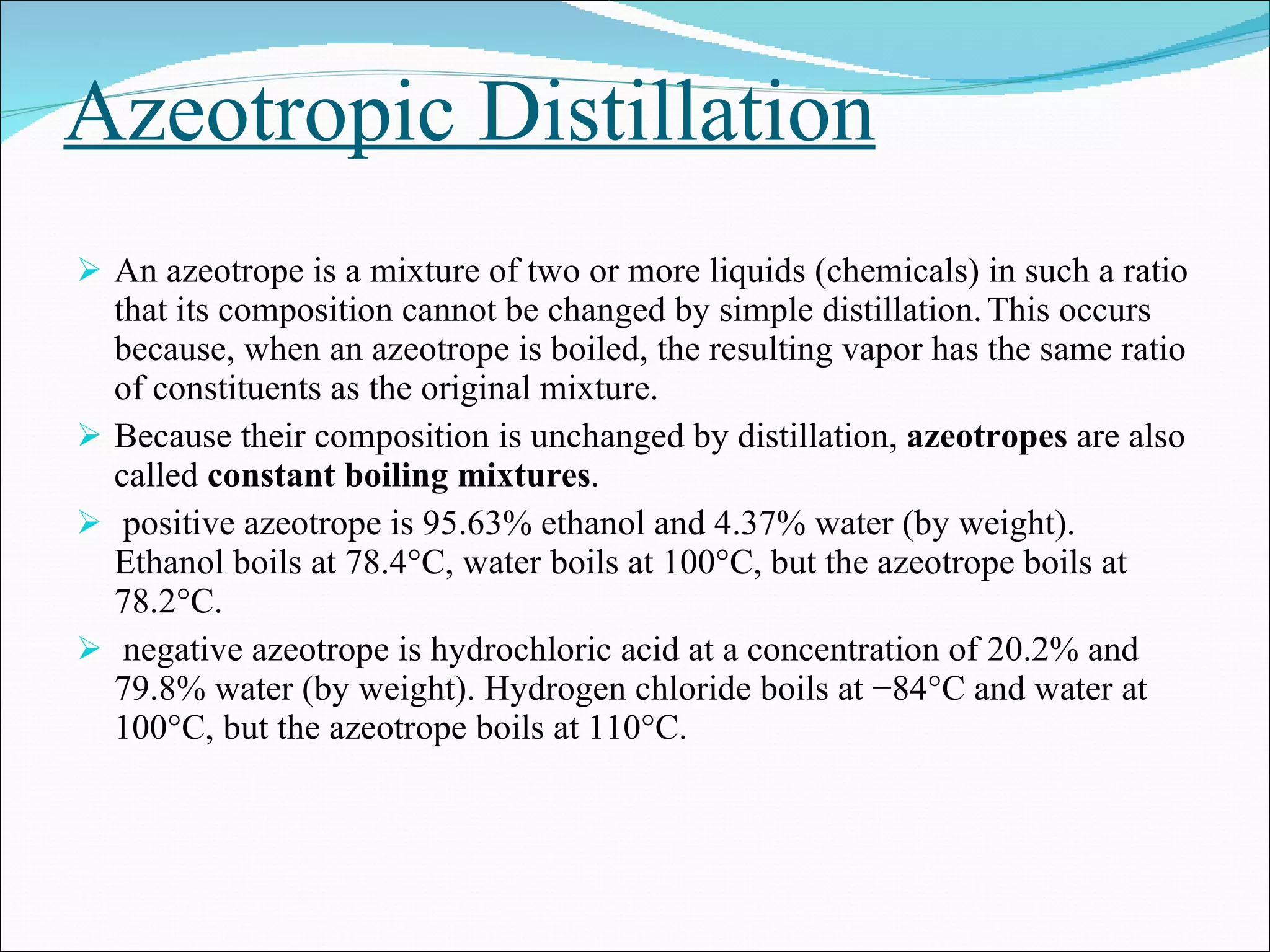
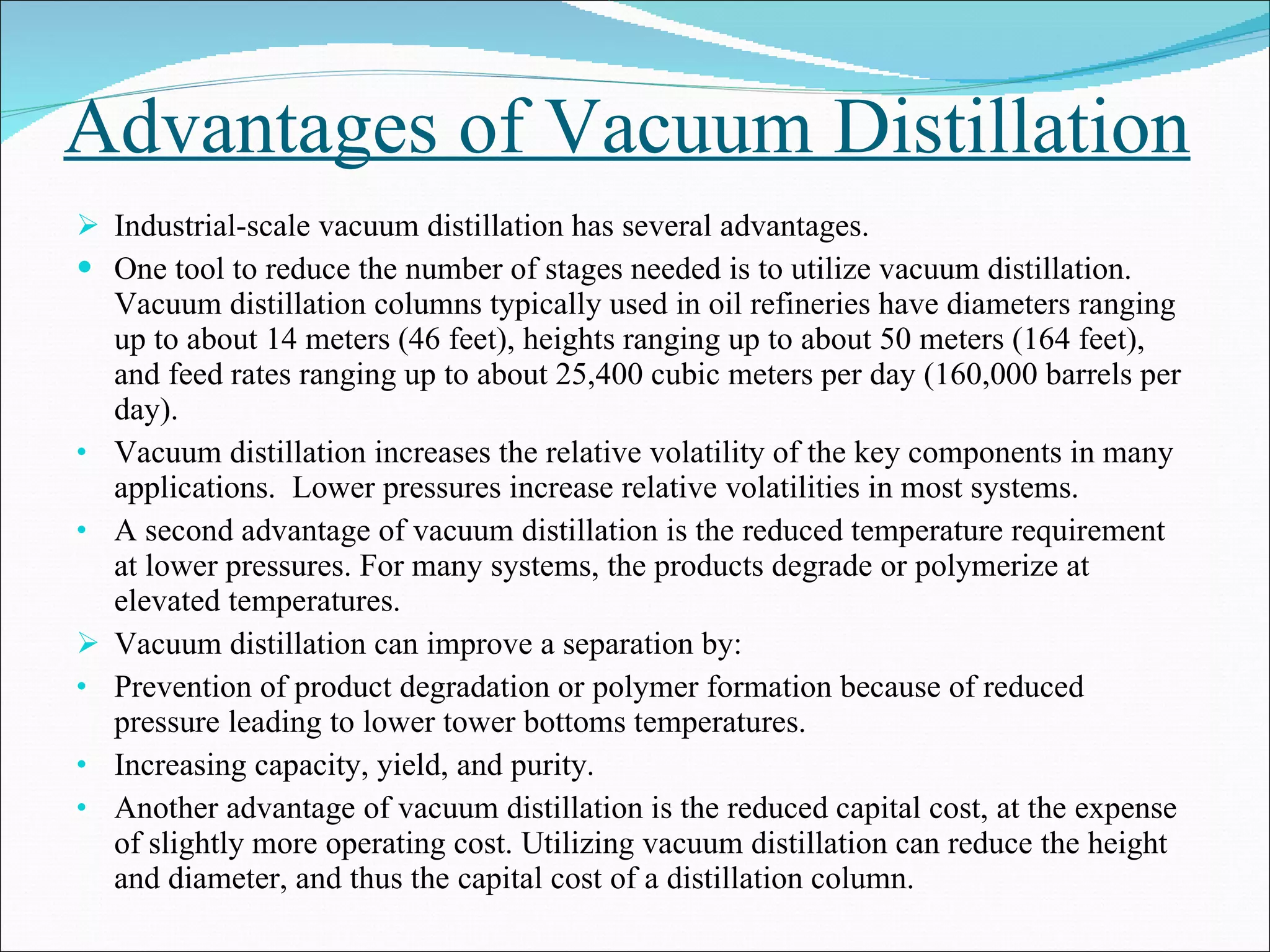
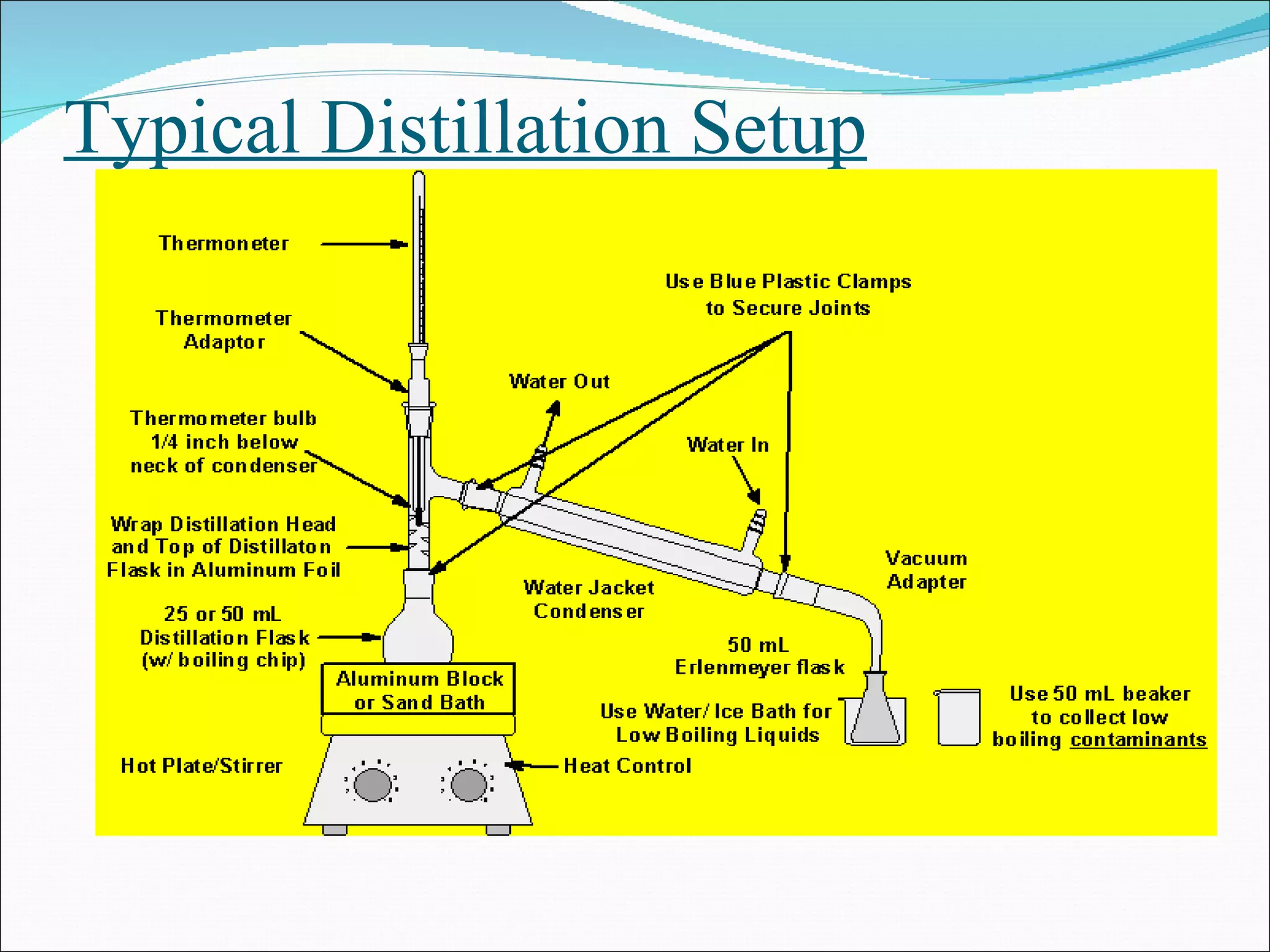
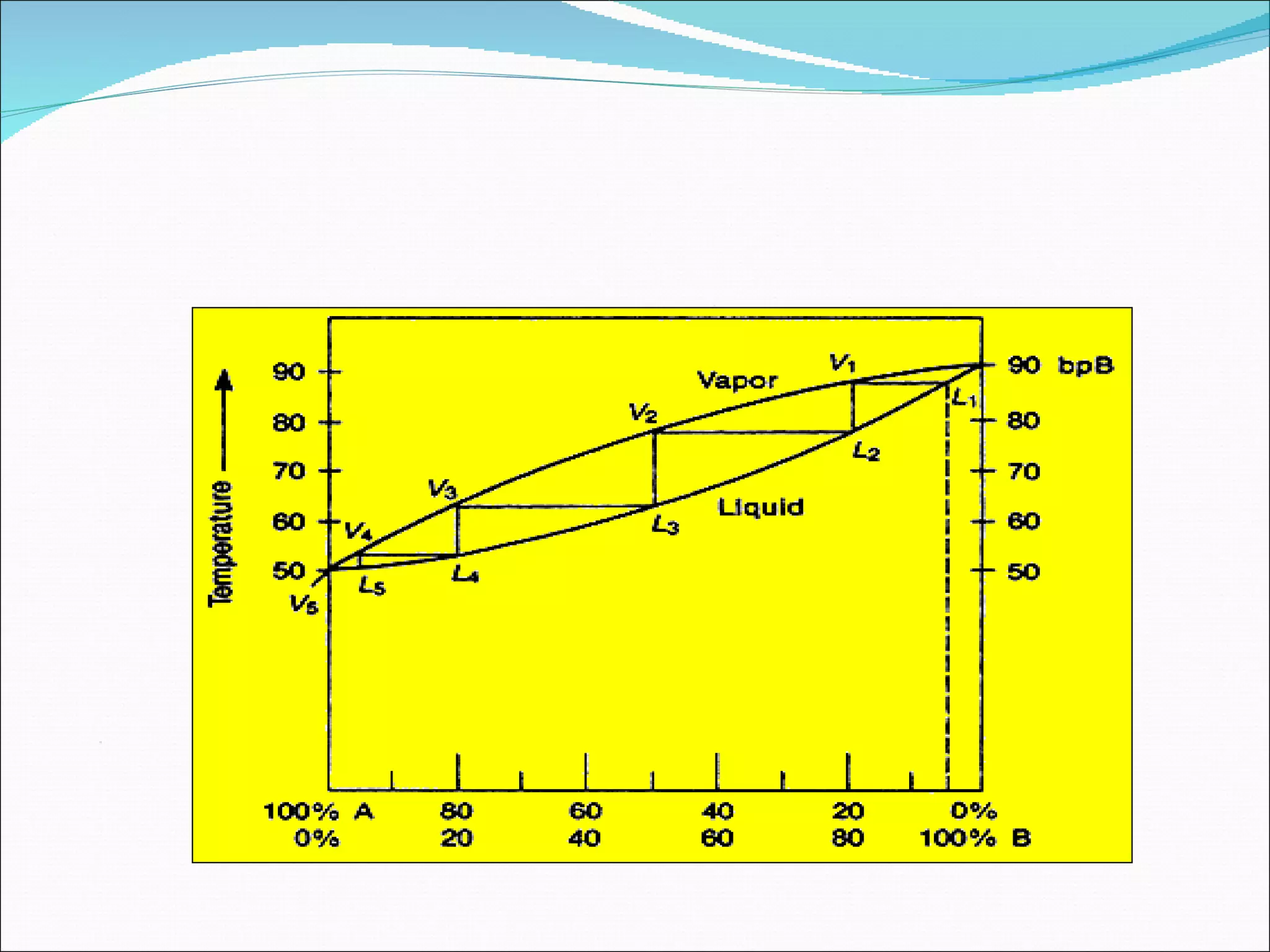
Distillation is a method to separate mixtures based on differences in volatility. It involves boiling the mixture and condensing the vapor produced. Simple distillation produces an impure distillate while fractional distillation uses a fractionating column for multiple vaporization-condensation cycles, allowing better separation. Vacuum distillation uses reduced pressure for distillation at lower temperatures to prevent degradation. Batch distillation processes mixtures in batches while continuous distillation constantly feeds and removes fractions.