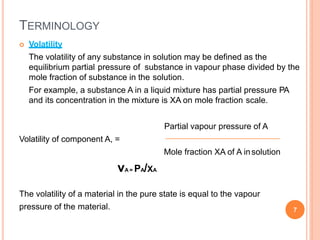
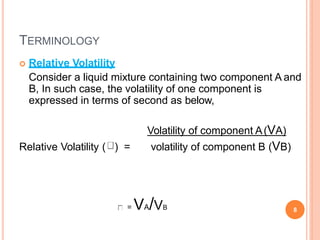
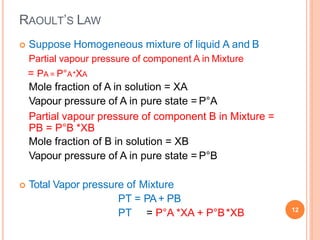
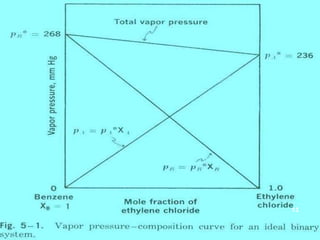
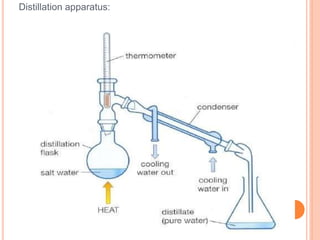
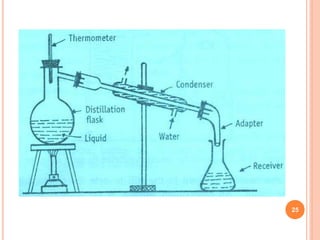
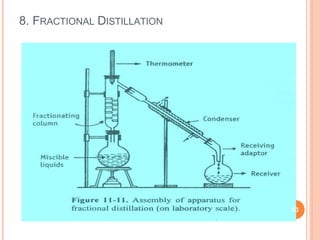
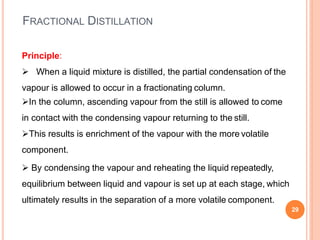
Distillation is a process that separates components of a liquid mixture based on differences in their volatilities. It involves boiling the mixture and condensing the vapor. Fractional distillation uses a fractionating column where partial condensation occurs, allowing some vapor to return to the still and enriching the remaining vapor in the more volatile component. This repeated condensation and vaporization results in a more effective separation than simple distillation.





















![FRACTIONAL DISTILLATION
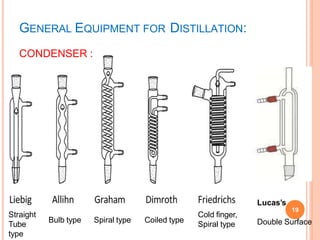
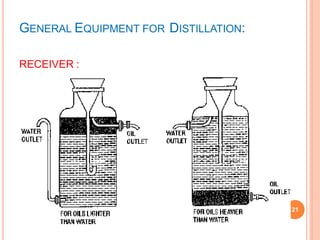
Fractionating columns Types
A] Packed columns
33](https://image.slidesharecdn.com/distillationchapter-221123153327-35e3a759/85/Distillation-chapter-pptx-33-320.jpg)



