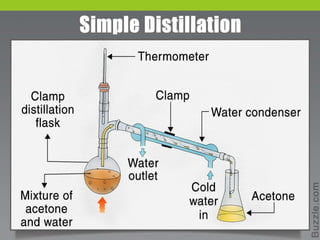
Distillation is a process used to separate liquids with different boiling points by heating a liquid mixture and collecting the vapor of the most volatile components. It works based on the principle that vapor pressure increases with temperature, allowing components to boil off one by one. Common applications include separating crude oil, purifying water, and producing alcoholic beverages. There are several types of distillation defined by factors like the temperature range, boiling point differences of components, and whether a vacuum is used. Simple distillation is suitable when components boil below 150°C and have at least a 25°C boiling point difference.