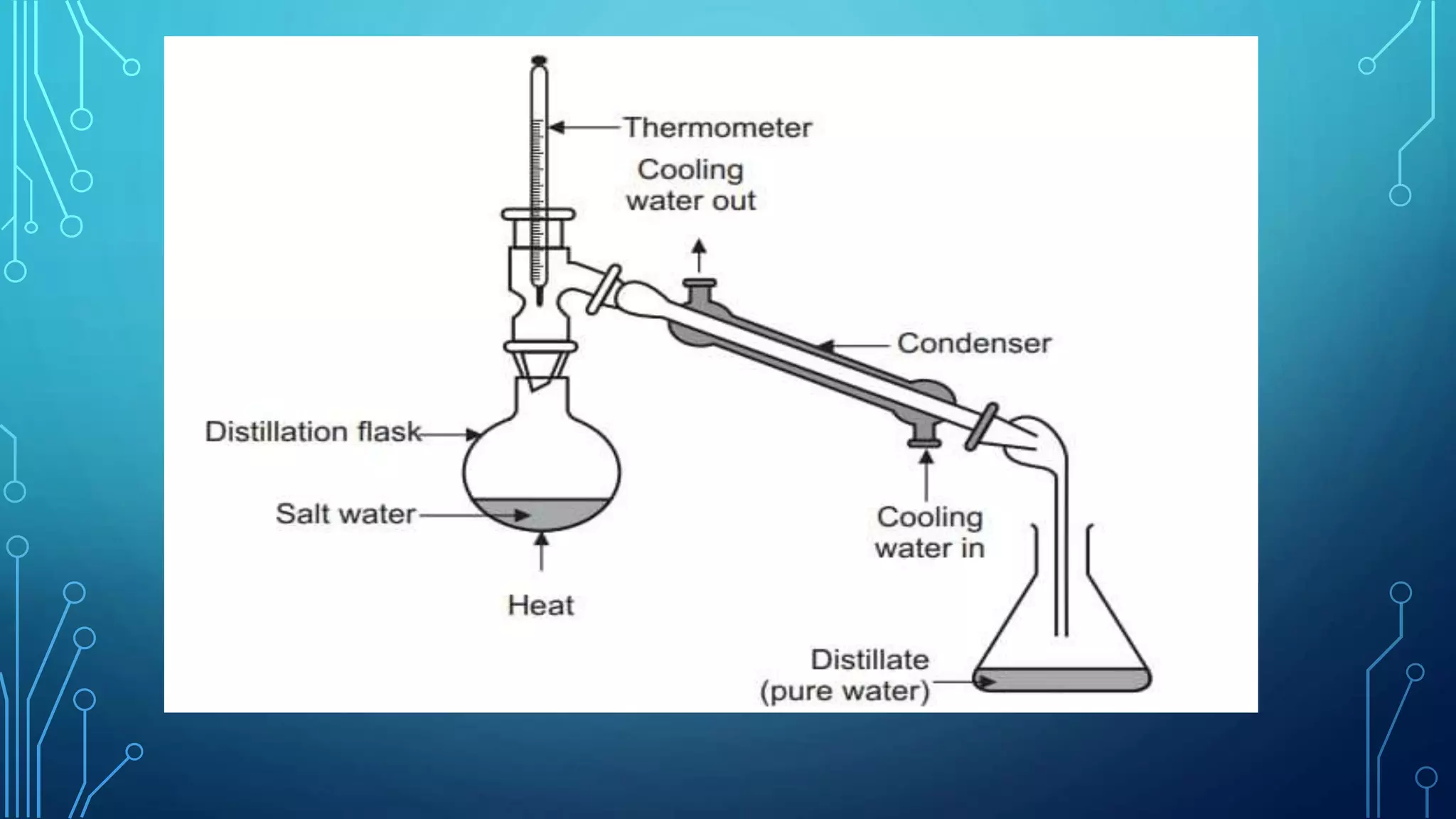
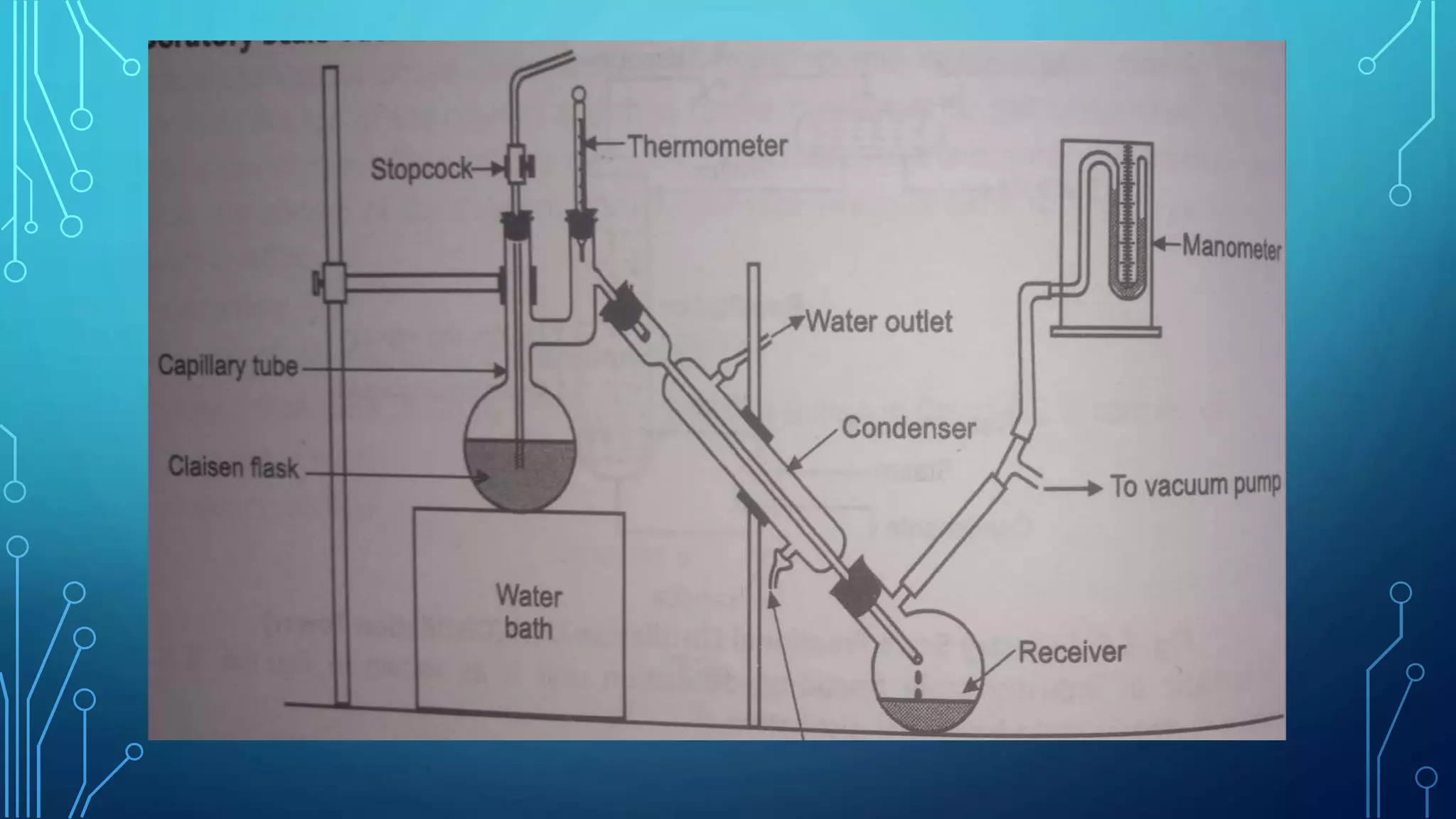
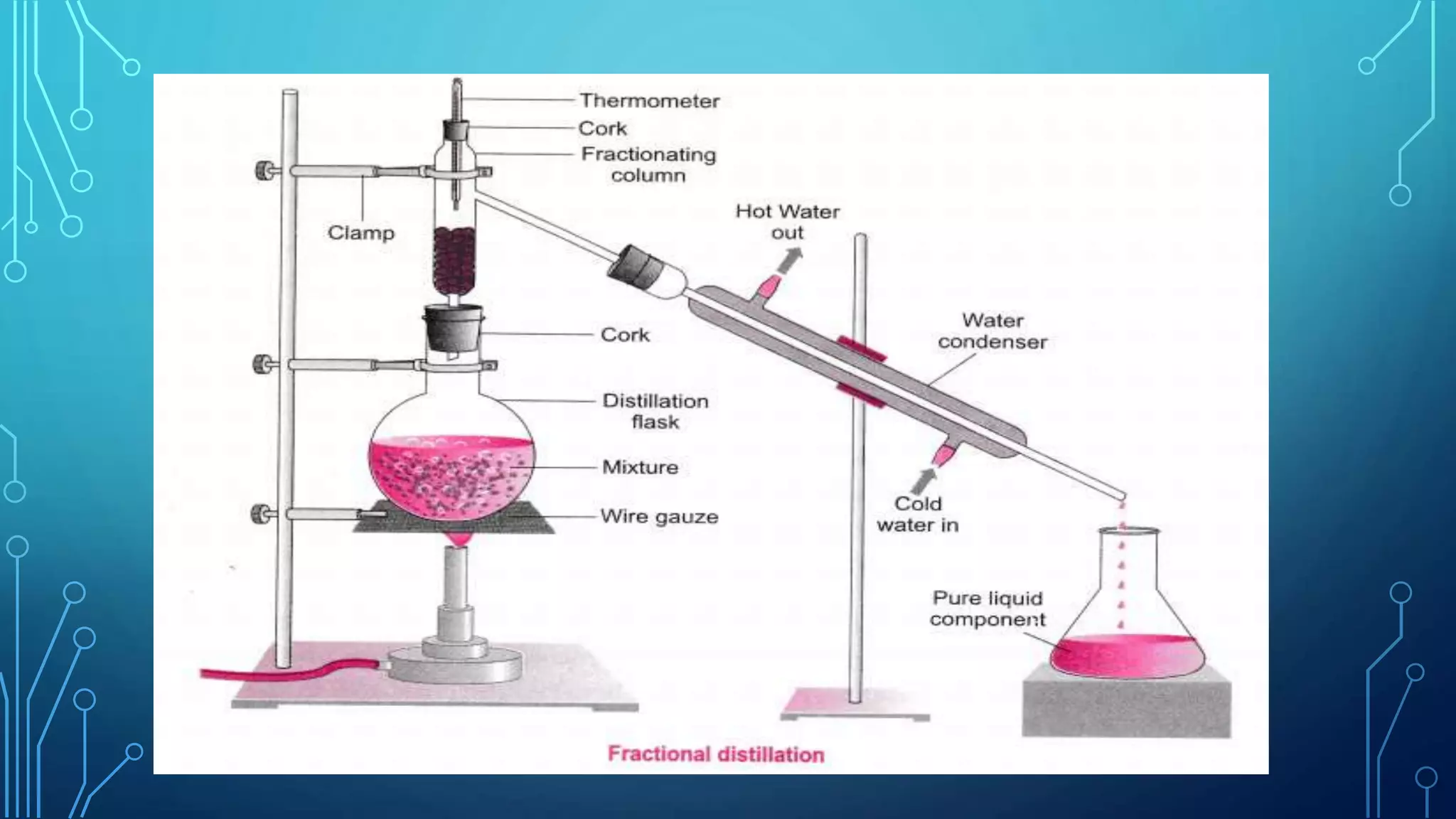
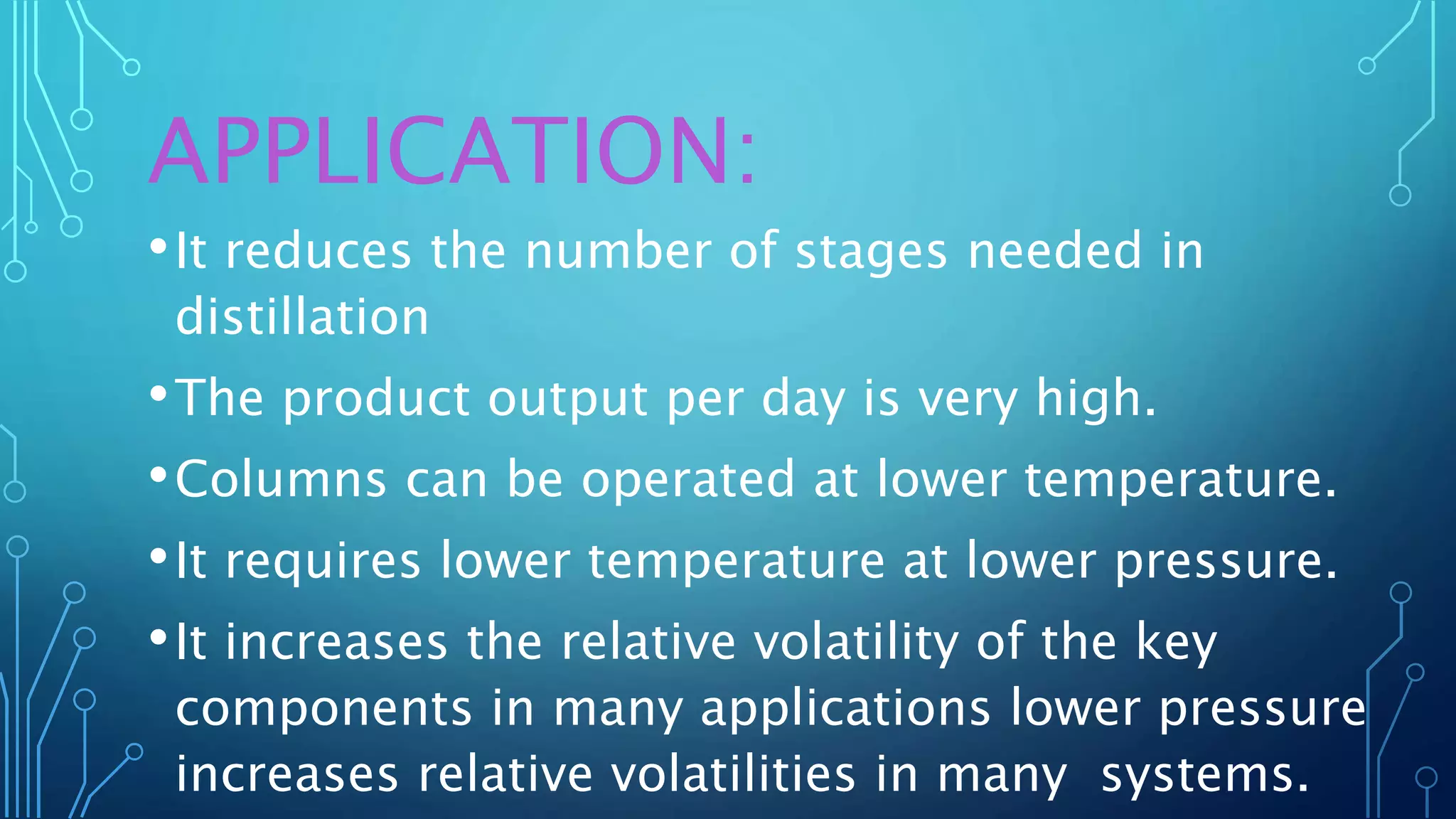
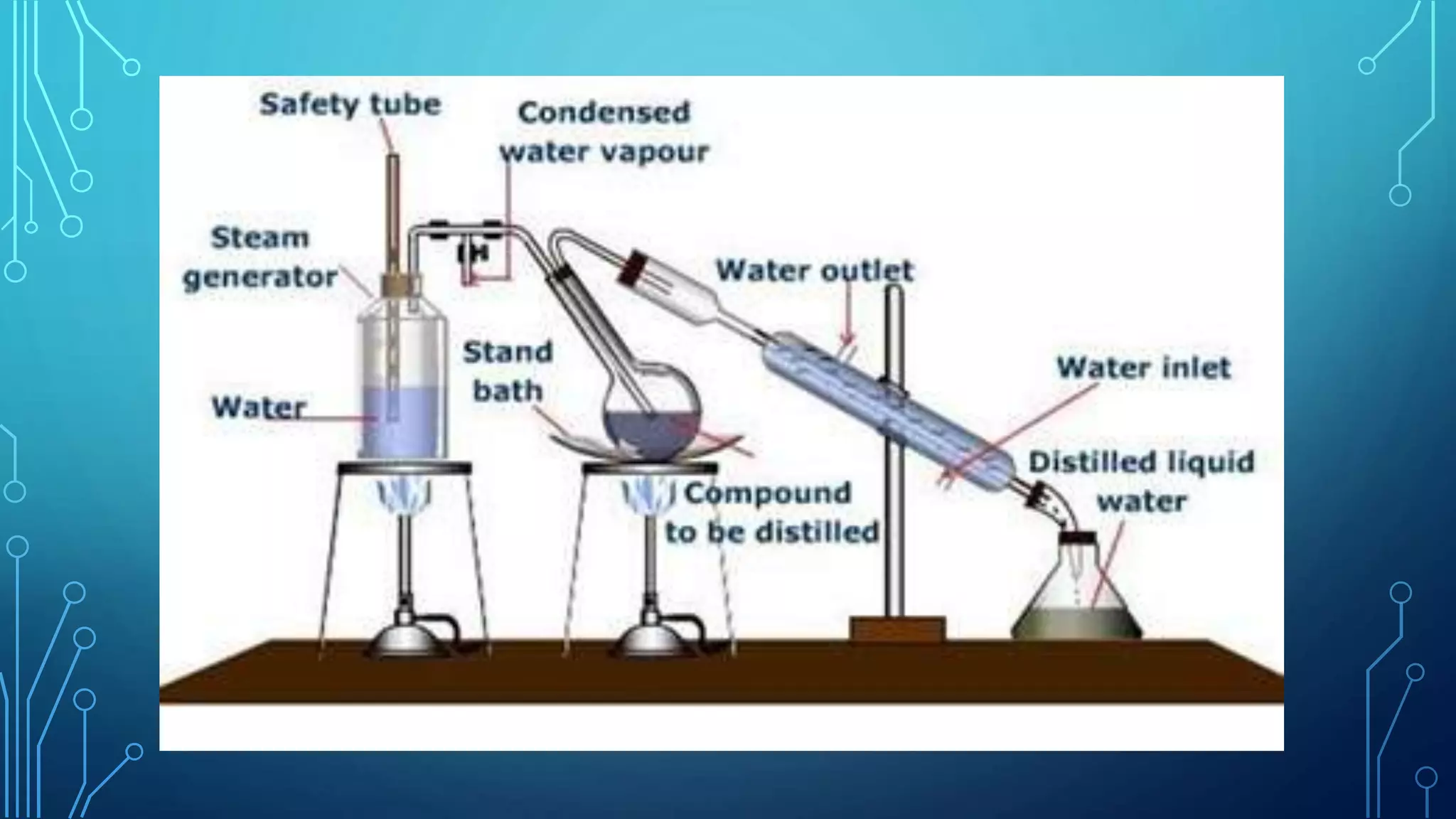
The document discusses distillation, a process for separating liquid mixtures based on different boiling points, and its various methods, including simple, fractional, vacuum, steam, and flash distillation. Each method is explained in terms of principles, applications, advantages, and disadvantages, emphasizing its role in purifying solvents and extracting essential oils. Additionally, the document outlines key theoretical concepts such as Raoult's law and the operation of distillation apparatus.