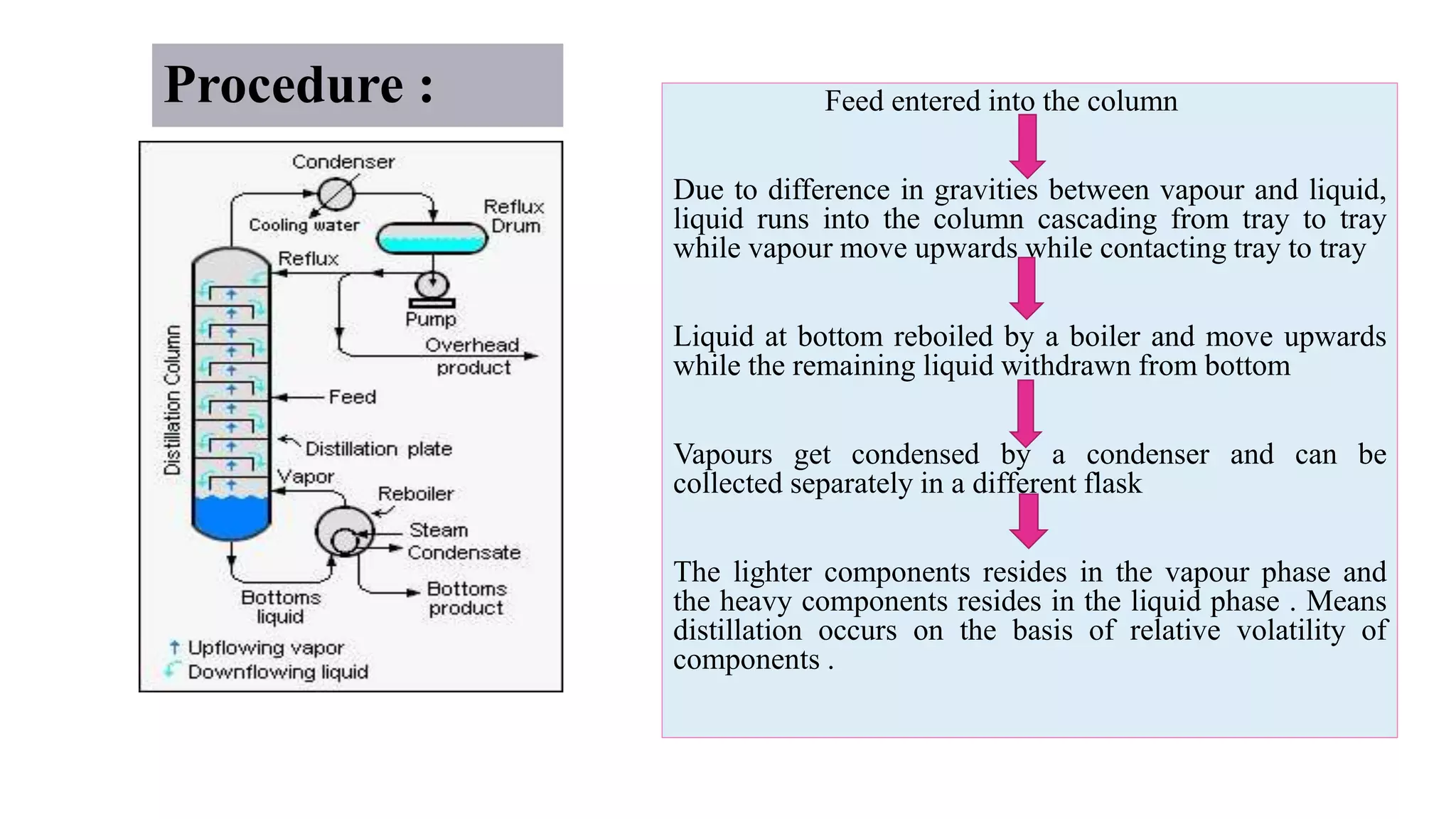
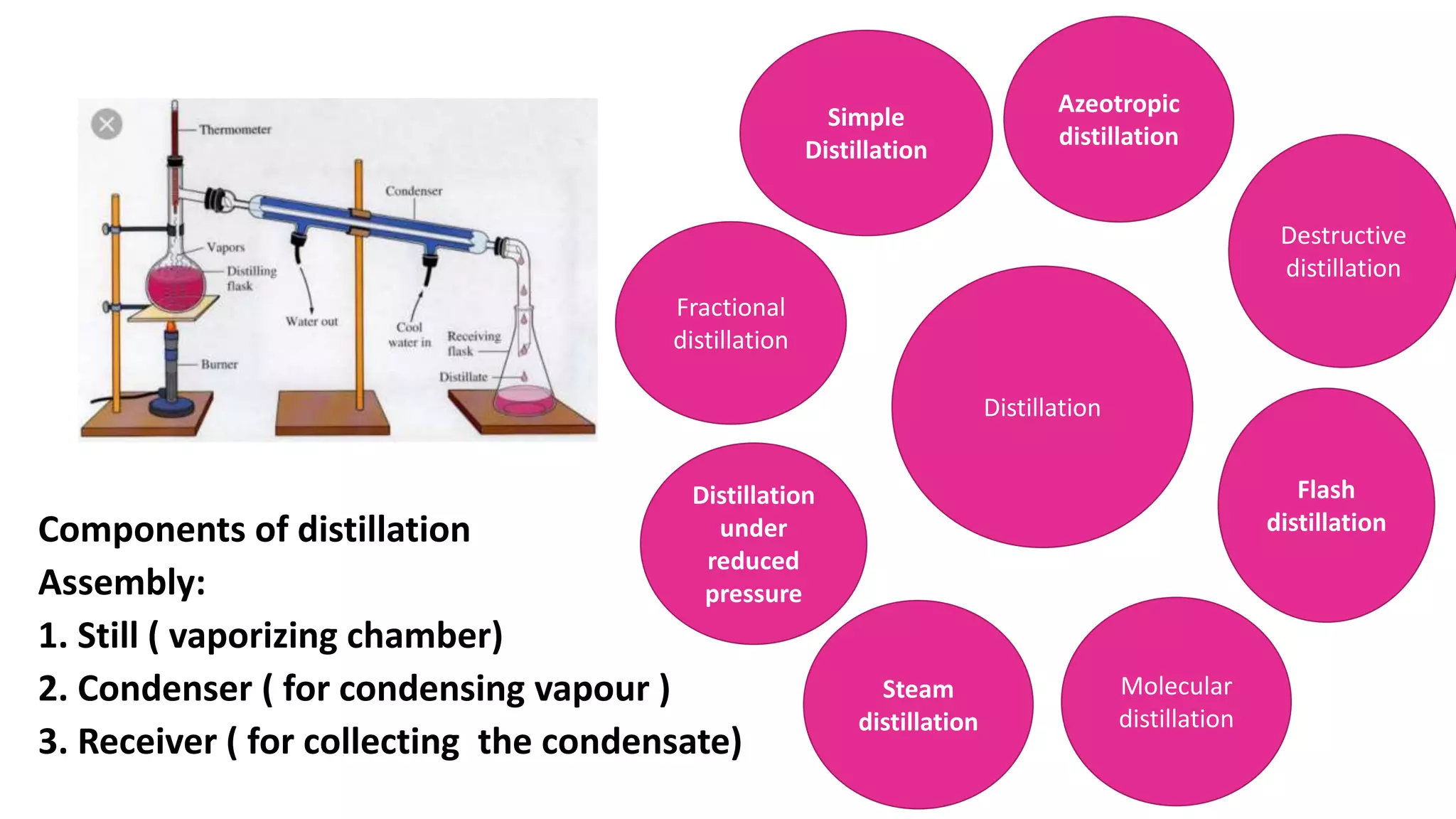
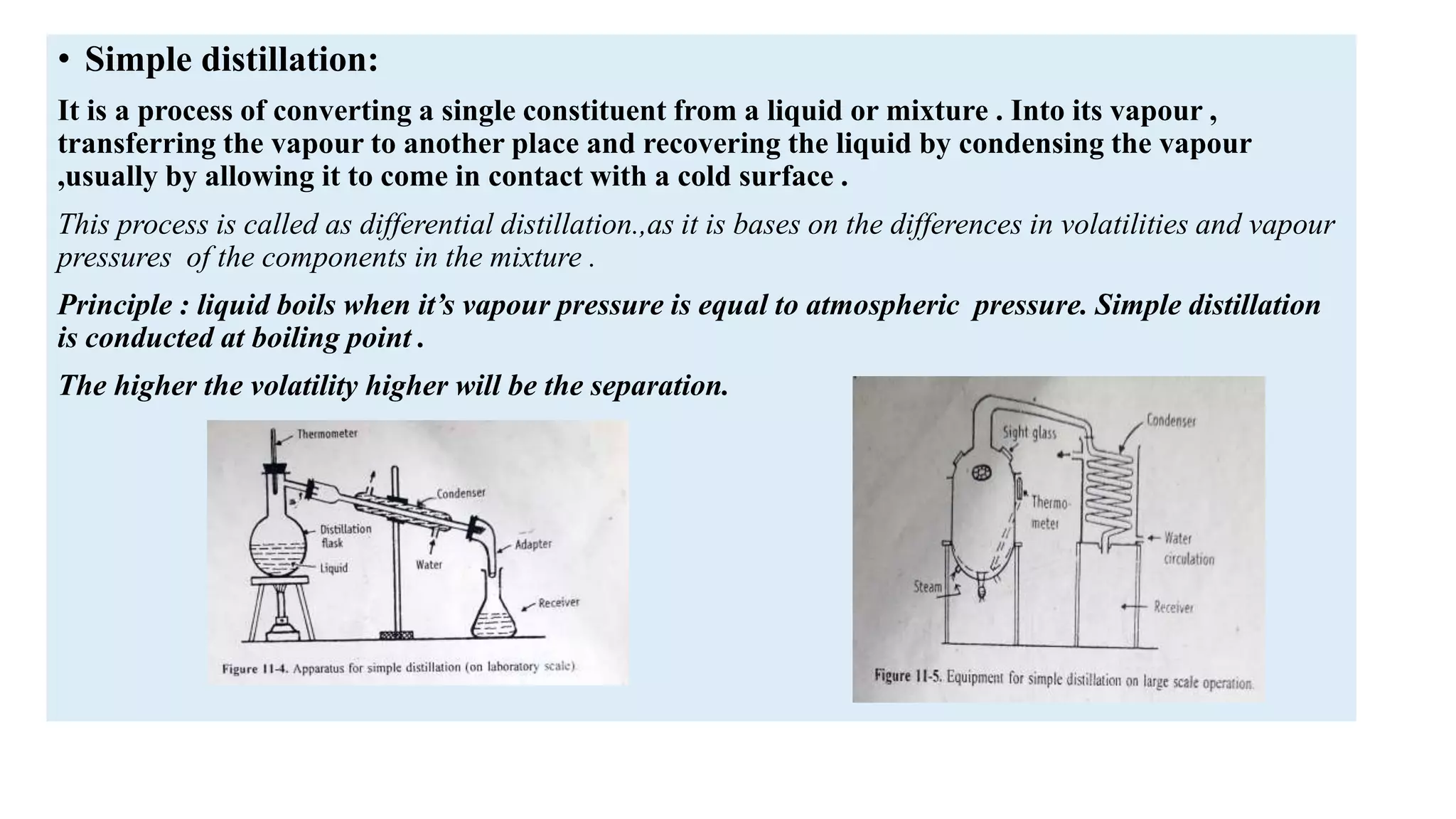
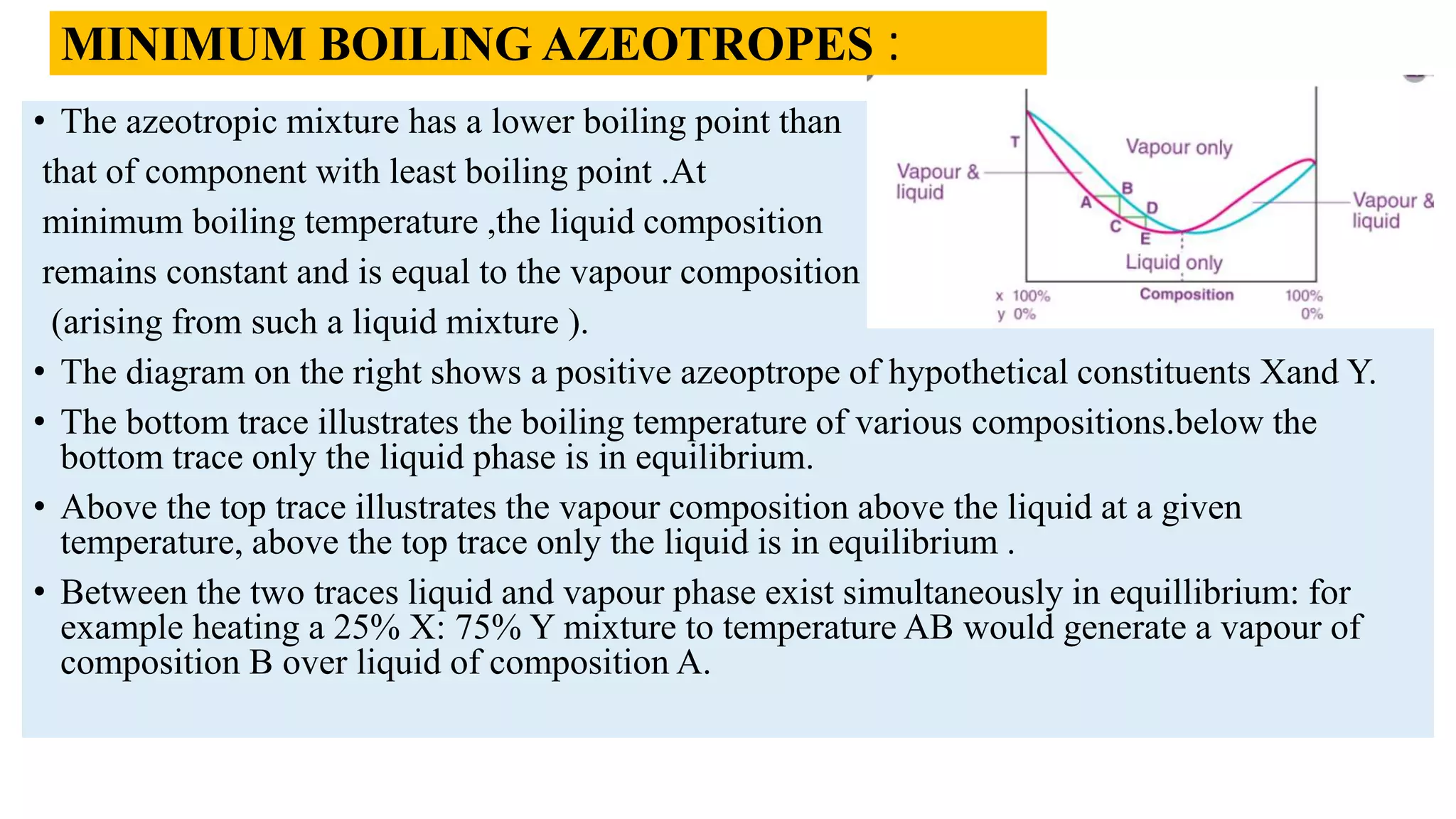
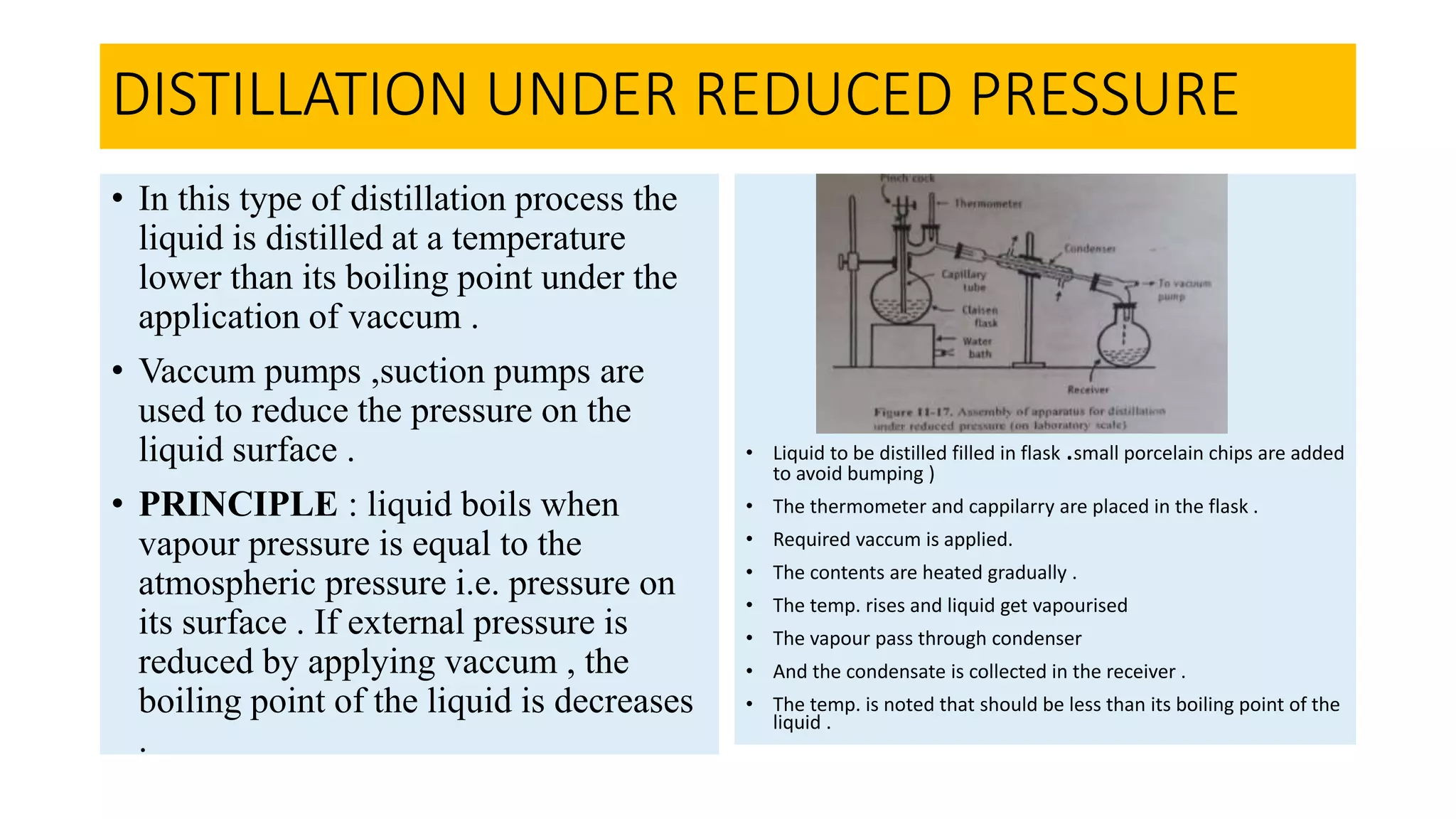
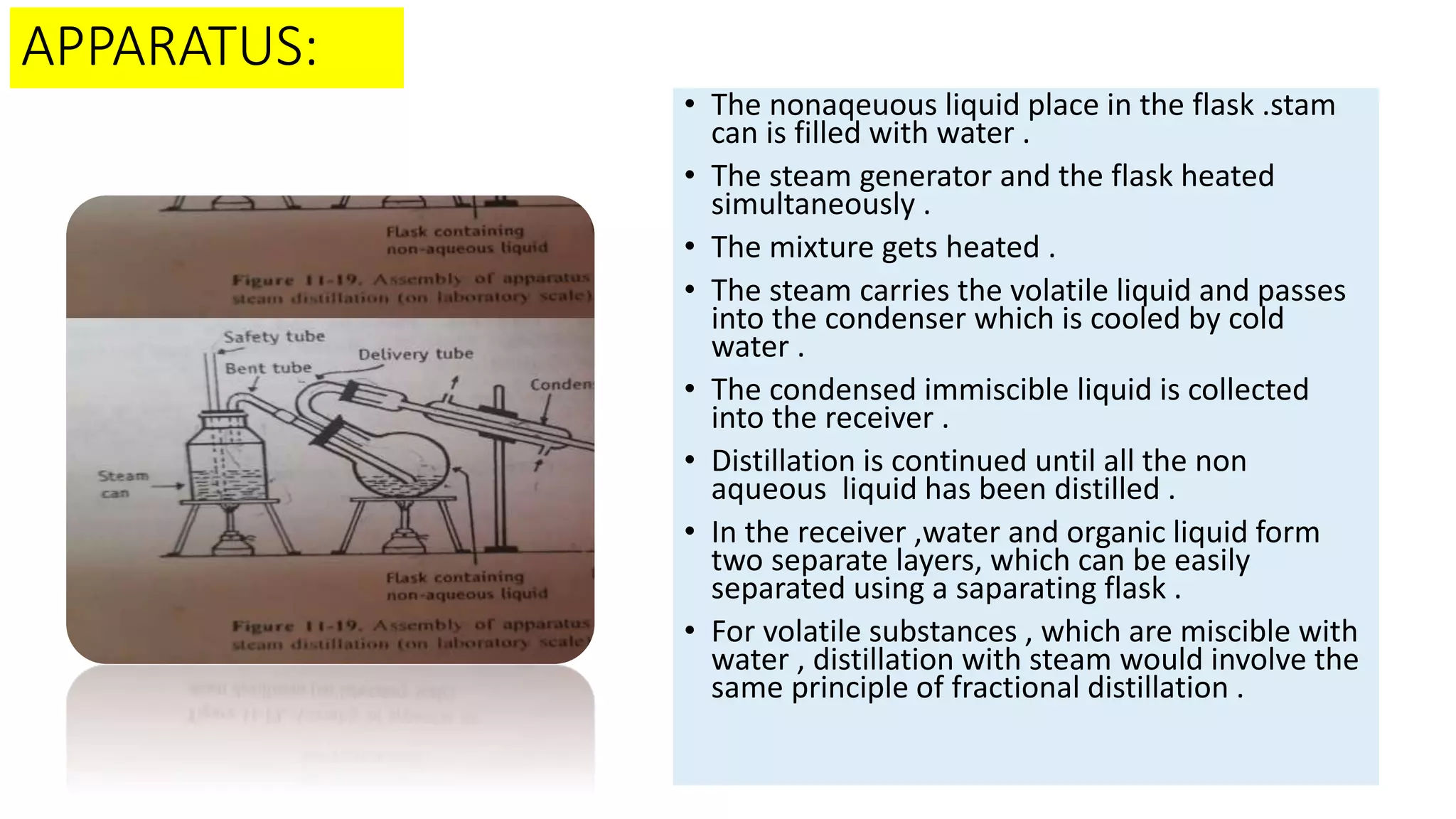
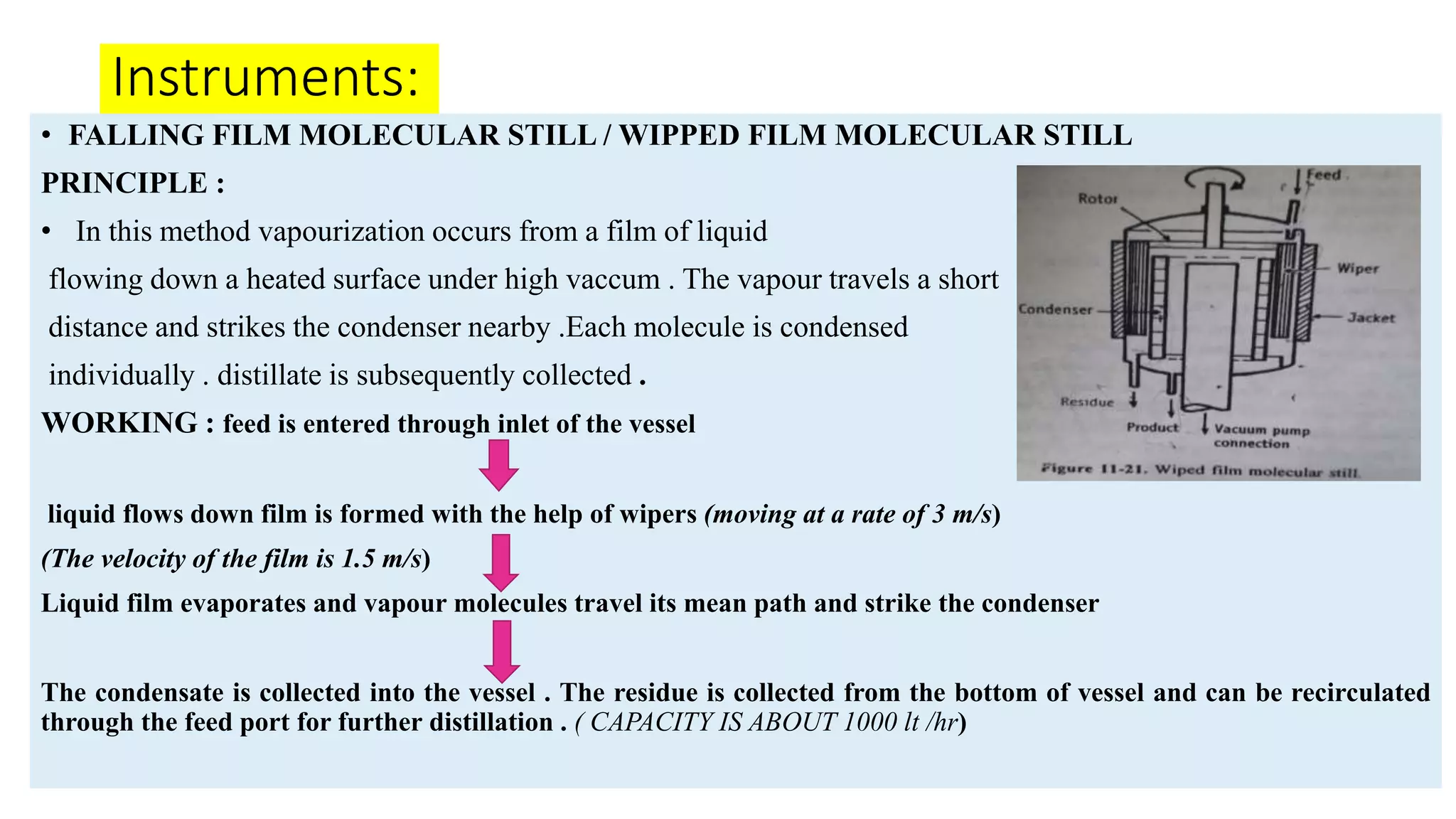
This PowerPoint presentation discusses various types of distillation processes including simple distillation, fractional distillation, flash distillation, azeotropic distillation, and steam distillation. It explains the basic principles and components of distillation, differences between continuous and batch distillation, factors that influence column efficiency, and challenges with separating azeotropic mixtures. Key concepts covered include Raoult's law, distillation curves, reflux ratios, and minimum and maximum boiling azeotropes.