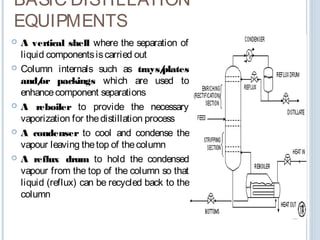
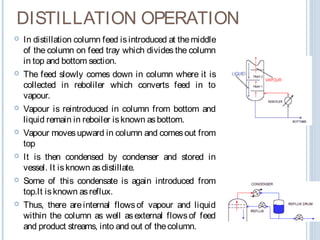
Distillation is a process that separates mixtures into individual components based on differences in their boiling points. It works by heating the mixture to vaporize components with lower boiling points, which are then cooled and condensed.
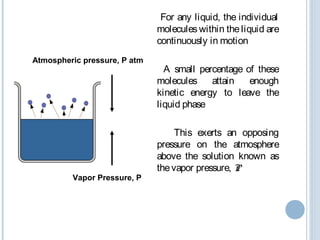
The key principles are that vapor pressure increases with temperature, allowing the lower boiling components to vaporize first. According to Raoult's law, the vapor produced will be enriched in the more volatile components compared to the liquid mixture.
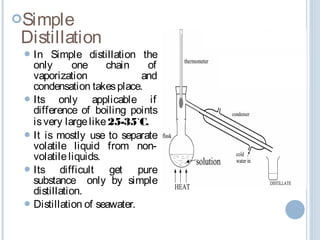
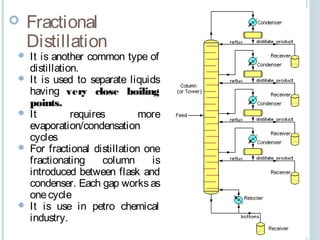
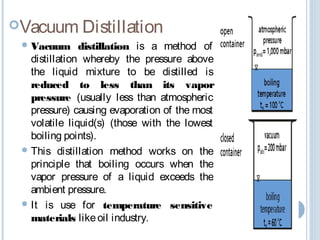
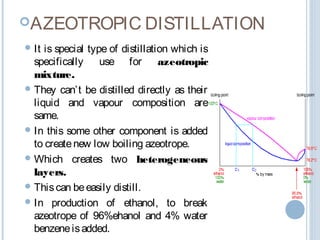
There are several types of distillation including simple, fractional, vacuum, and azeotropic distillation. Simple distillation is used when components have very different boiling points while fractional distillation with multiple stages is needed for similar boiling points. Vacuum distillation lowers the