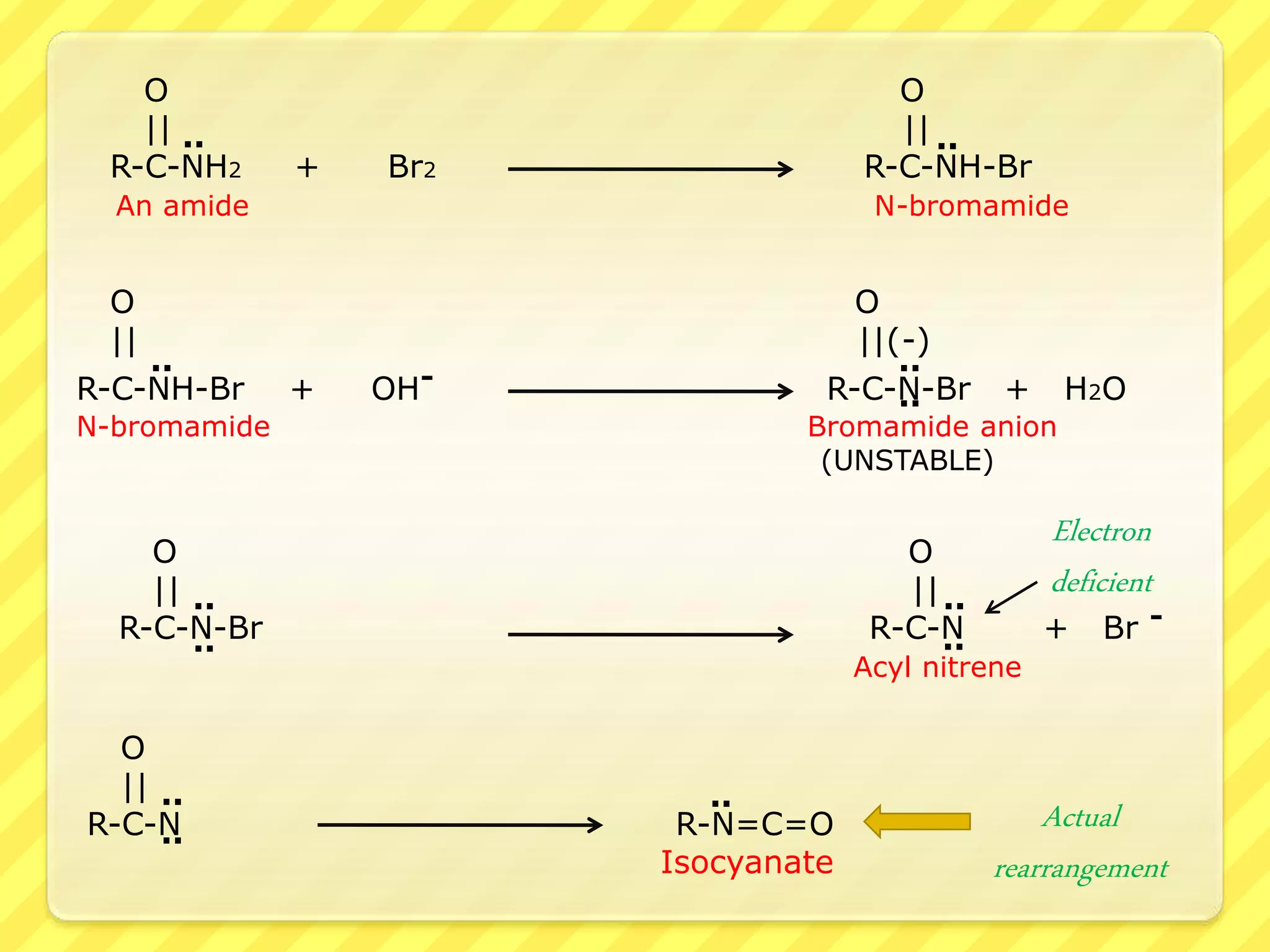
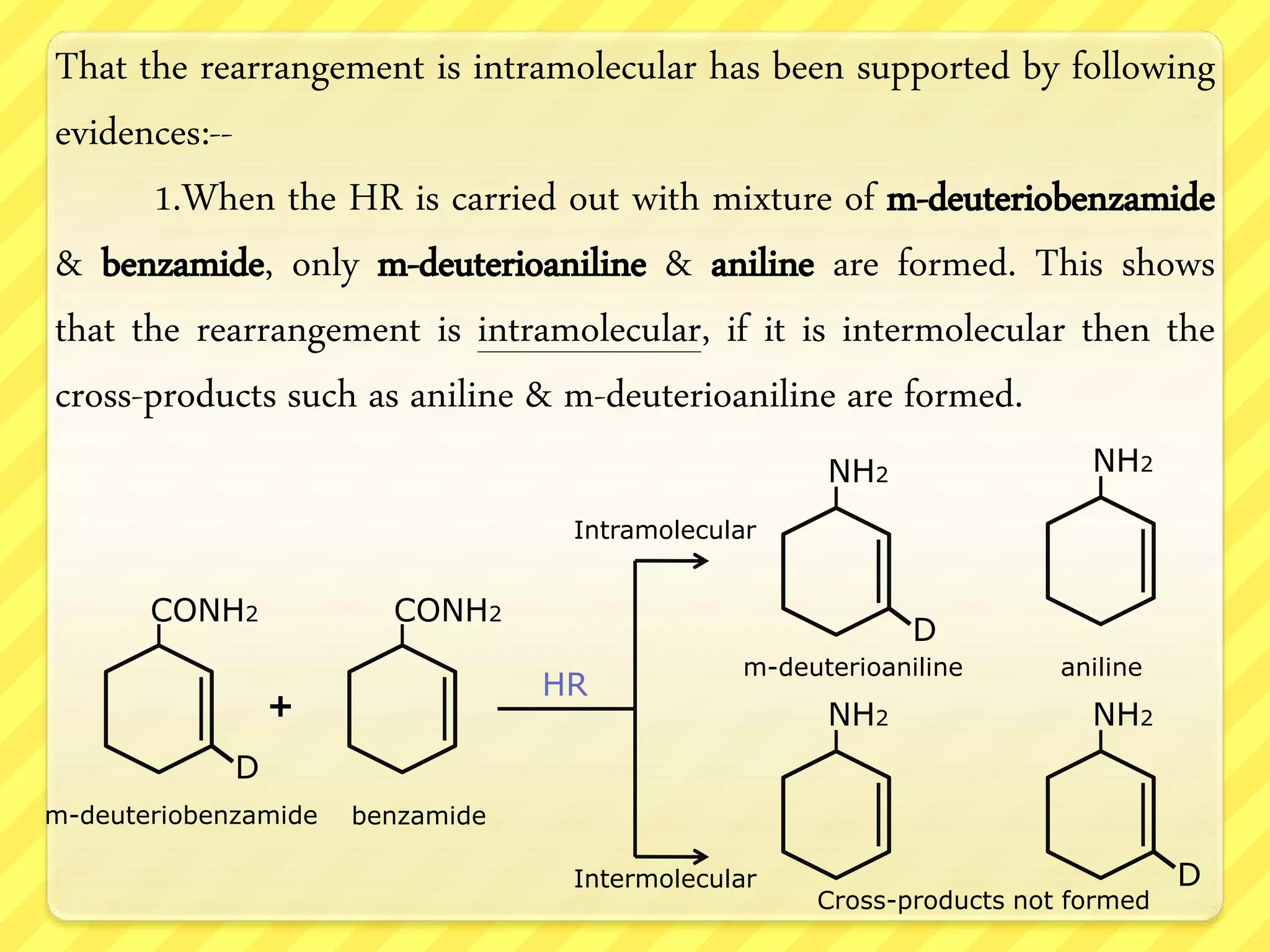
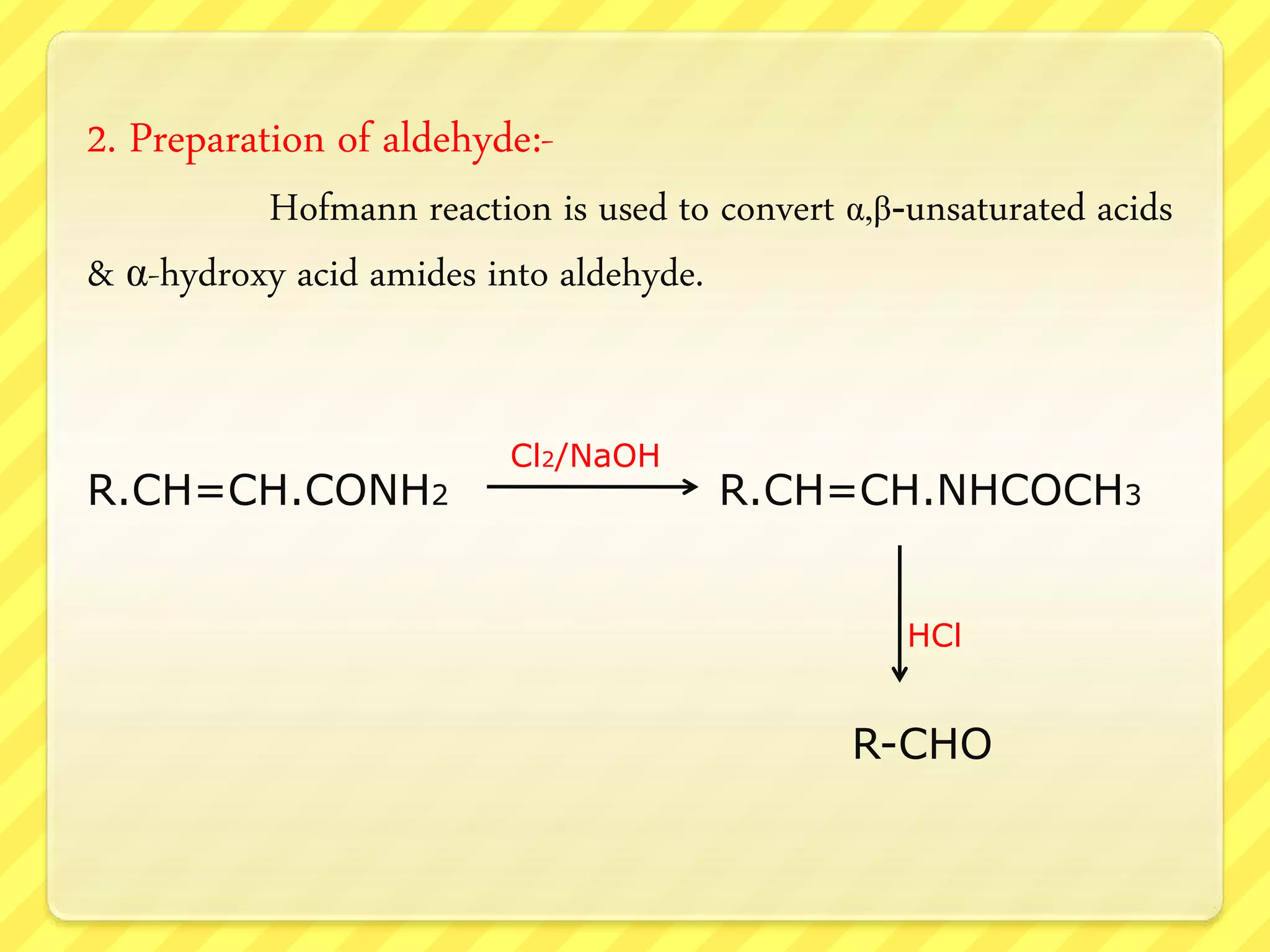
The Hofmann rearrangement is a chemical reaction that converts amides into primary amines with one less carbon atom using alkaline hypohalite or bromine in an alkaline medium. The process involves multiple steps, including bromination, rearrangement, and hydrolysis, retaining the stereochemistry of migrating groups if they are chiral. This reaction has practical applications in synthesizing various amines and aldehydes from carboxylic acids and their derivatives.