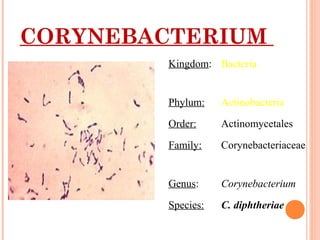
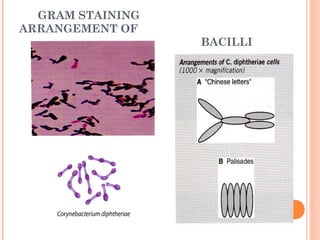
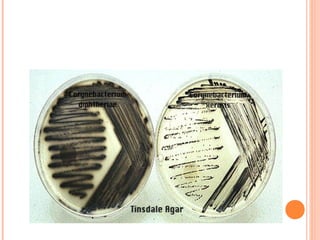
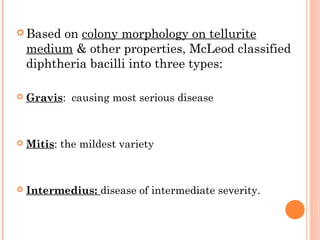
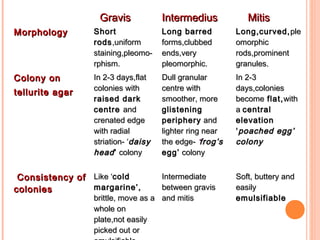
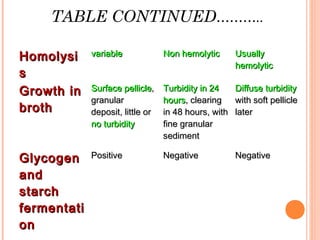
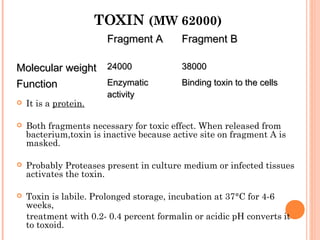
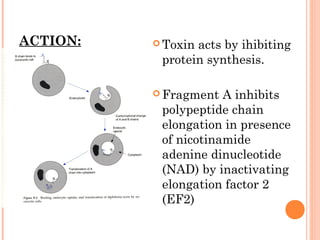
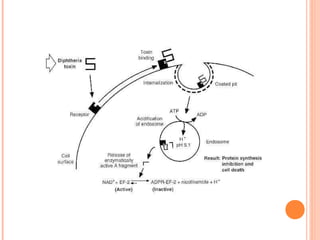
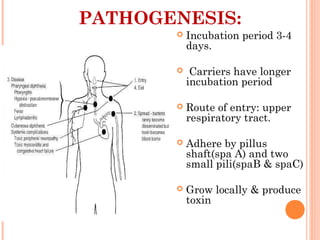
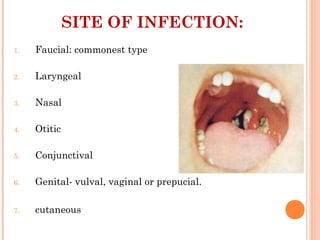
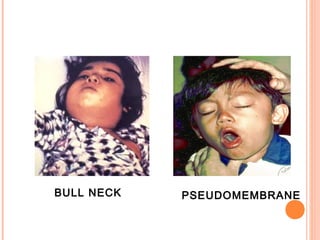
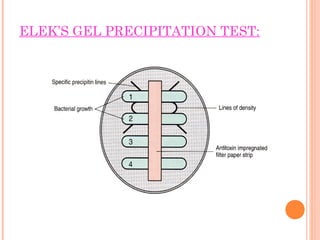
Corynebacterium diphtheriae is a gram-positive bacterium that causes diphtheria. It is transmitted through respiratory droplets and produces a potent exotoxin. The exotoxin inhibits protein synthesis and causes severe illness. Active immunization with diphtheria toxoid vaccine provides protection. Laboratory diagnosis involves isolating C. diphtheriae from lesions and confirming toxin production. Treatment involves antitoxin administration and antibiotics like penicillin. Complications can include respiratory obstruction, paralysis, and death.