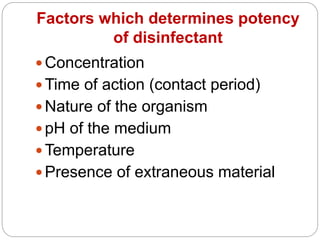
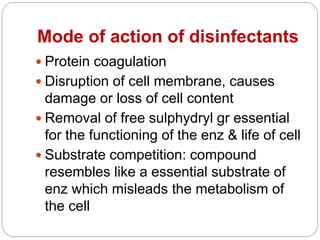
The document discusses various chemical agents used for disinfection. It describes the ideal properties of disinfectants and factors affecting their potency. Various classes of chemical disinfectants are explained - alcohols, aldehydes, dyes, halogens, phenols, gases, surface active agents and metallic salts. Specific chemicals from each class are mentioned along with their mechanisms and uses. Methods of testing disinfectants are also briefly covered.