Embed presentation
Downloaded 314 times
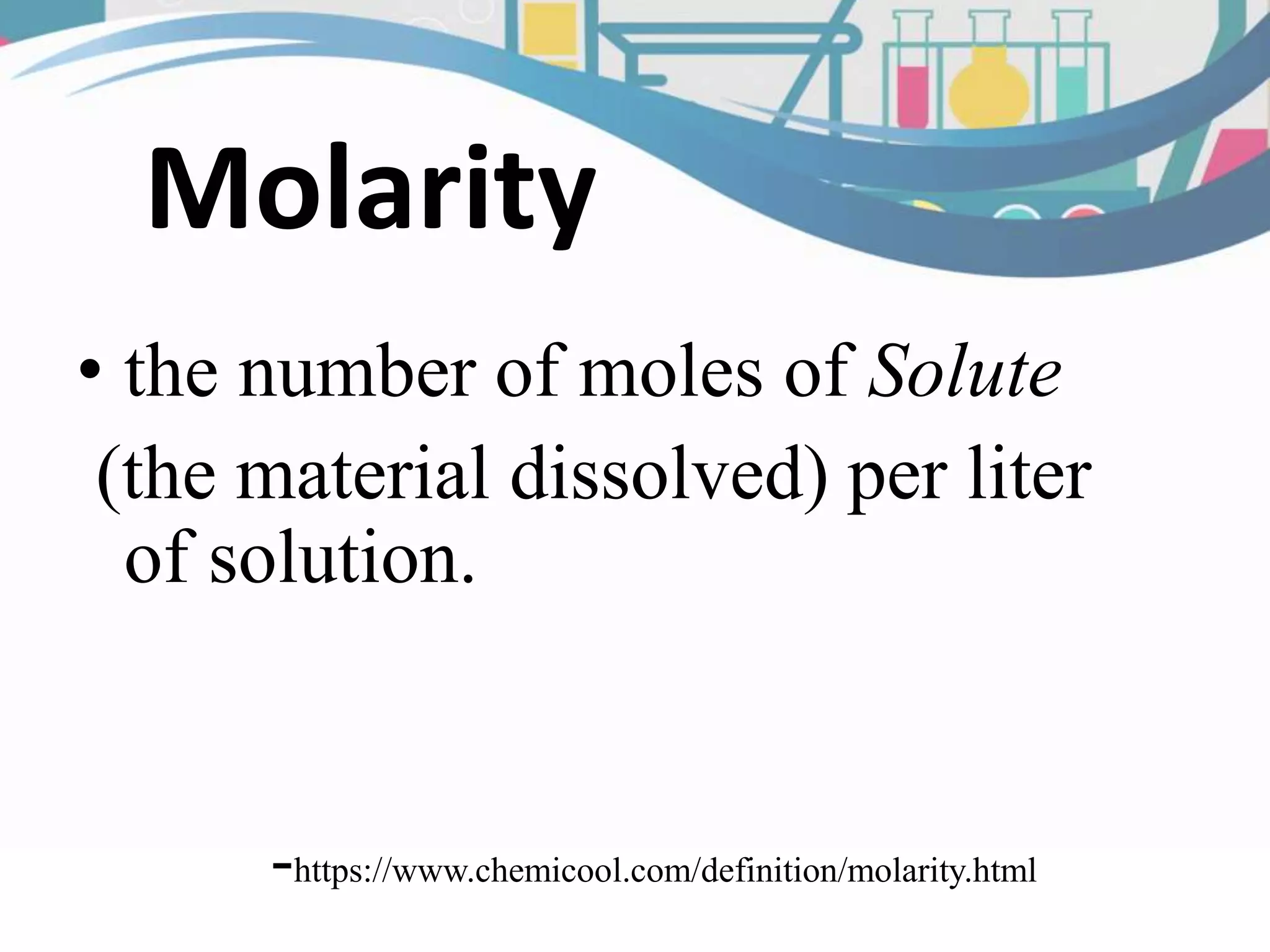


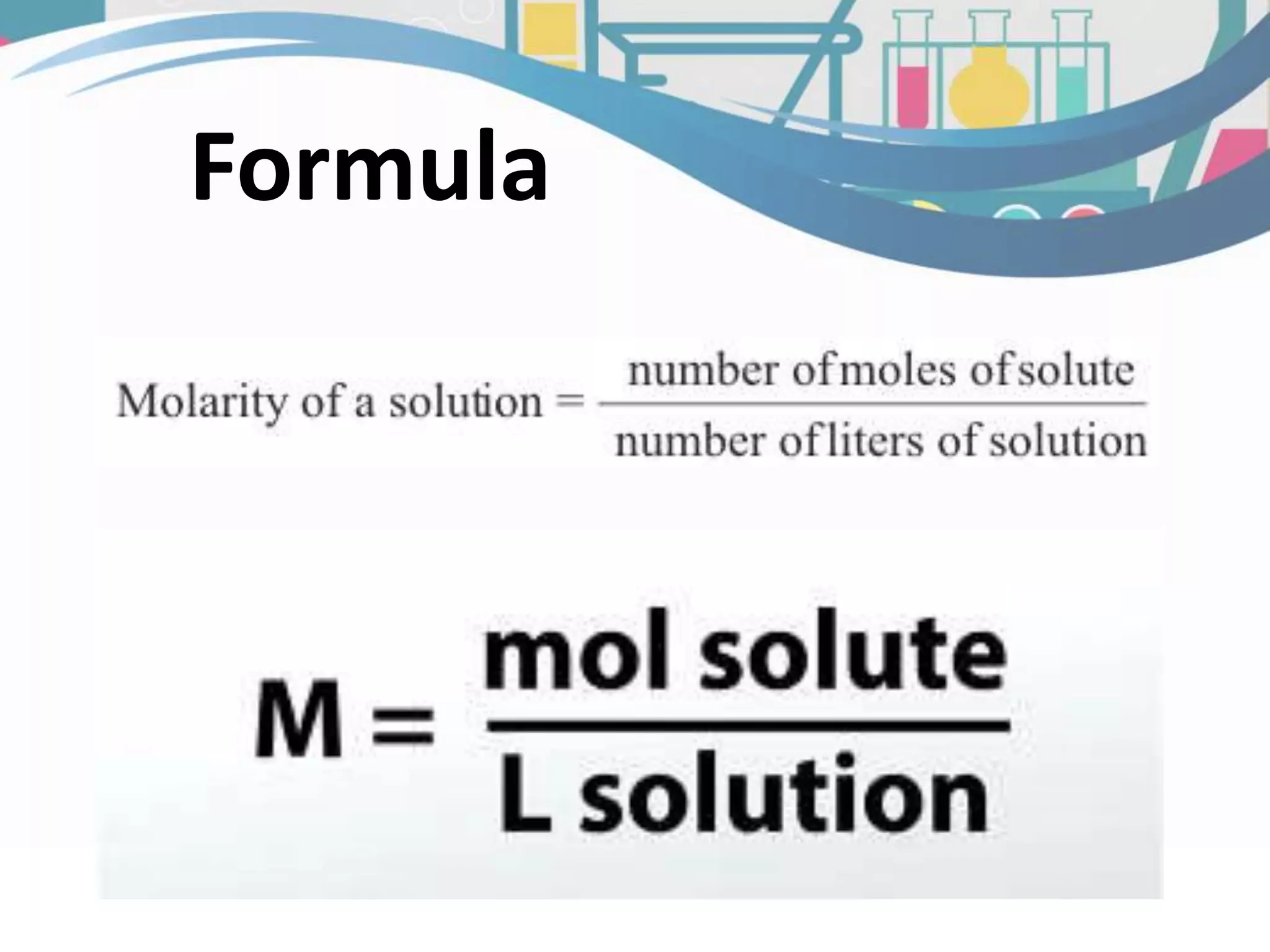
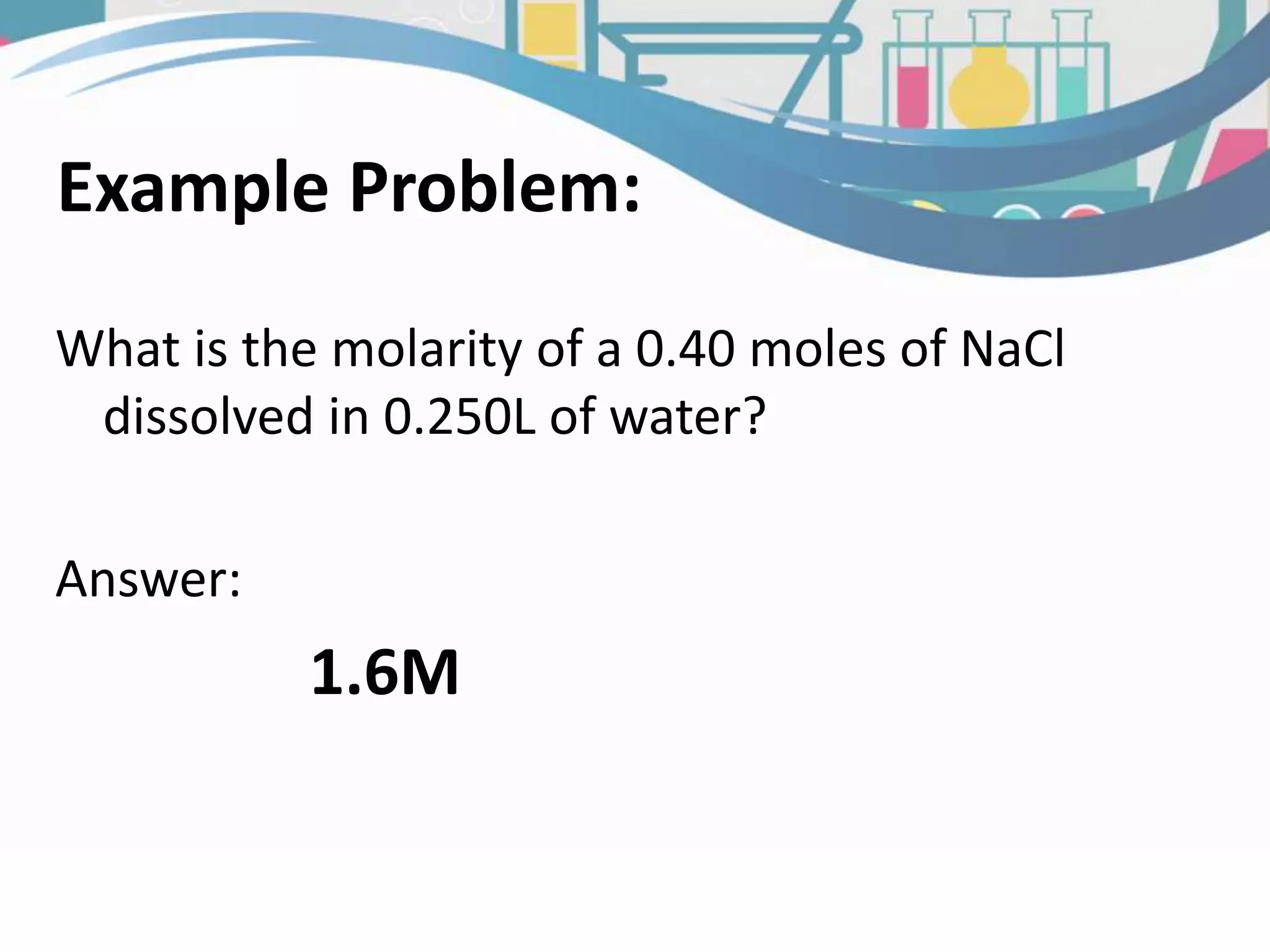
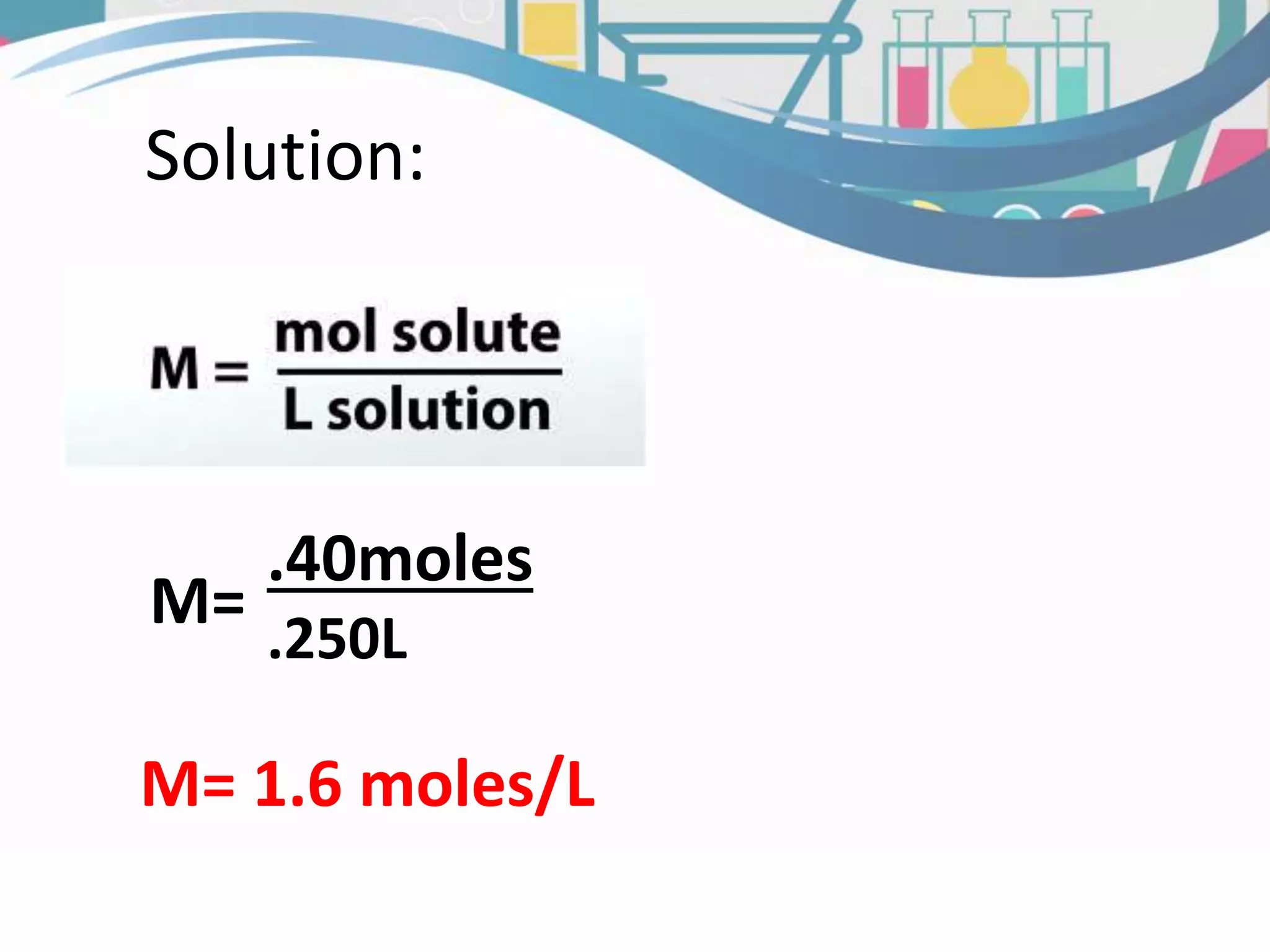


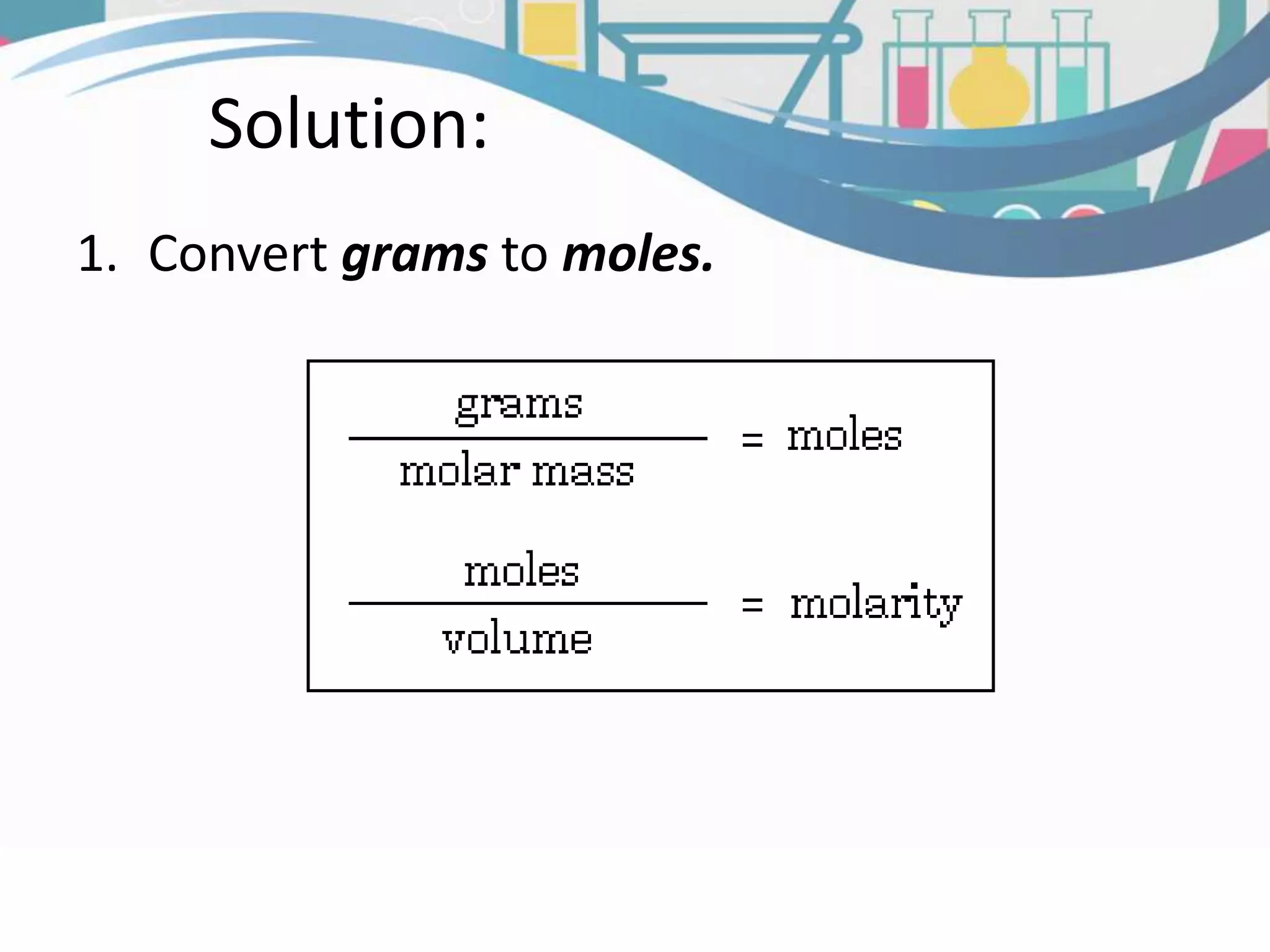





This document discusses molarity, which is defined as the number of moles of solute per liter of solution. Molarity is calculated by taking the moles of solute and dividing by the total liters of solution. The document provides an example calculation of molarity using 0.40 moles of NaCl dissolved in 0.250L of water, and gives a sample problem of calculating the molarity of 58.44 grams of NaCl dissolved in 2.00L of solution. Two practice problems are included at the end to test the reader's understanding of calculating molarity.














