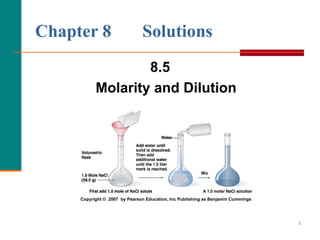
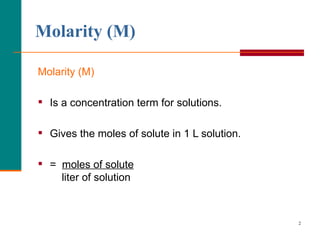
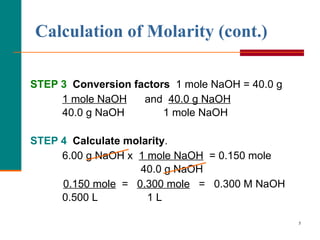
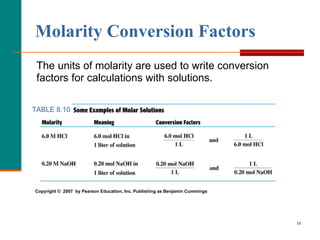
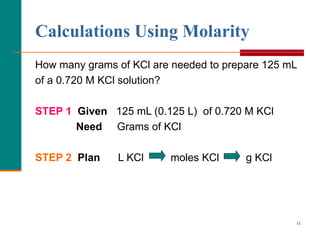
The document discusses molarity, which is a measurement of concentration that expresses the number of moles of solute per liter of solution. It provides examples of calculating molarity when given the mass of a solute and volume of solution. It also covers diluting solutions, where the moles of solute stay the same but the concentration decreases as volume increases. Molarity can be used to determine the moles, mass, or volume of a solution component when any two pieces of information are known.