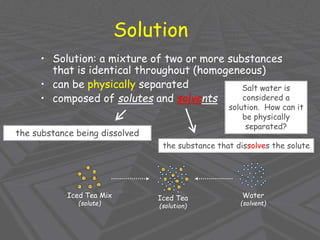
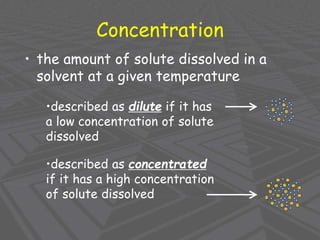
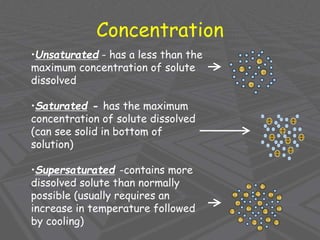
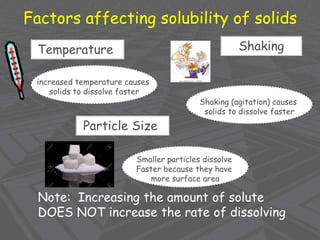
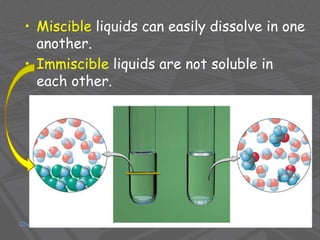
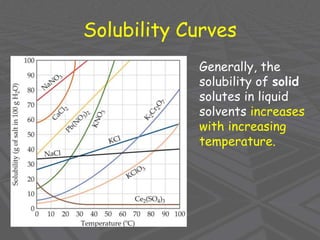
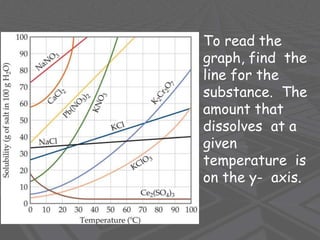
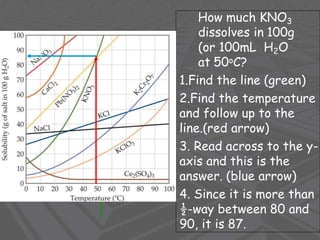
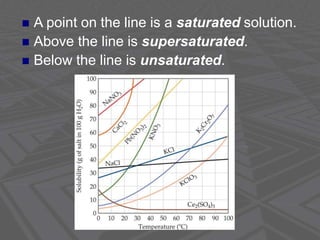
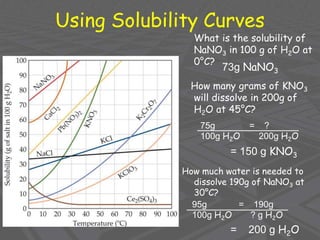
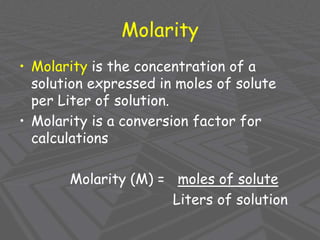
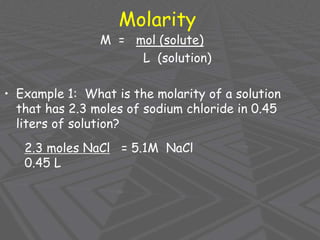
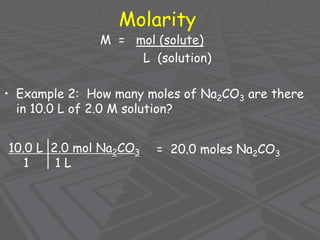
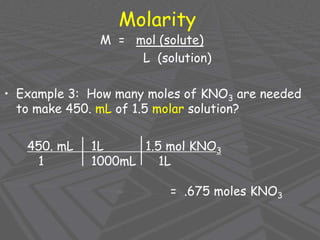
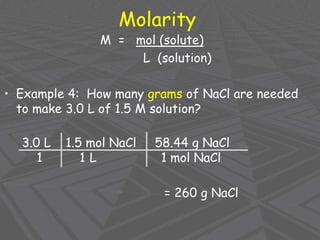
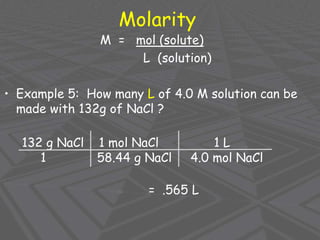
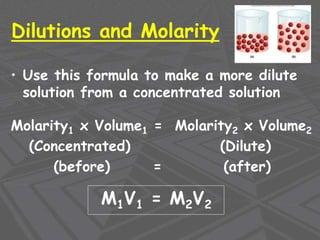
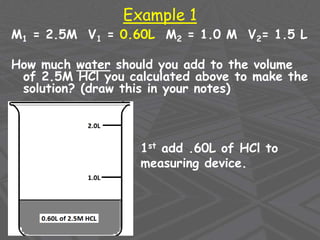
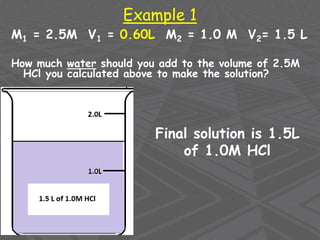
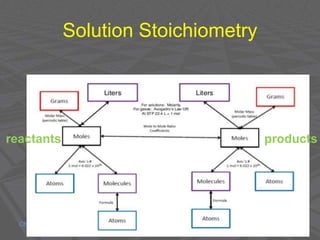
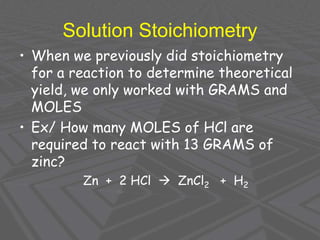
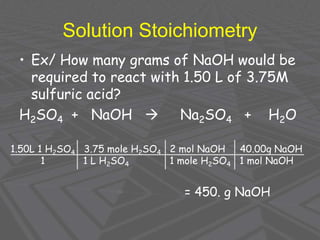
This document provides an overview of solutions and solubility. It defines key terms like solute, solvent, solution, homogeneous mixture, heterogeneous mixture, concentration, saturation, and factors that affect solubility. It also discusses quantitative concepts such as molarity, percent by mass, and how to use stoichiometry to calculate amounts in solutions. Specifically, it explains how to calculate amounts of solutes and solvents needed using molarity, percent by mass, and mole ratios from balanced chemical equations.