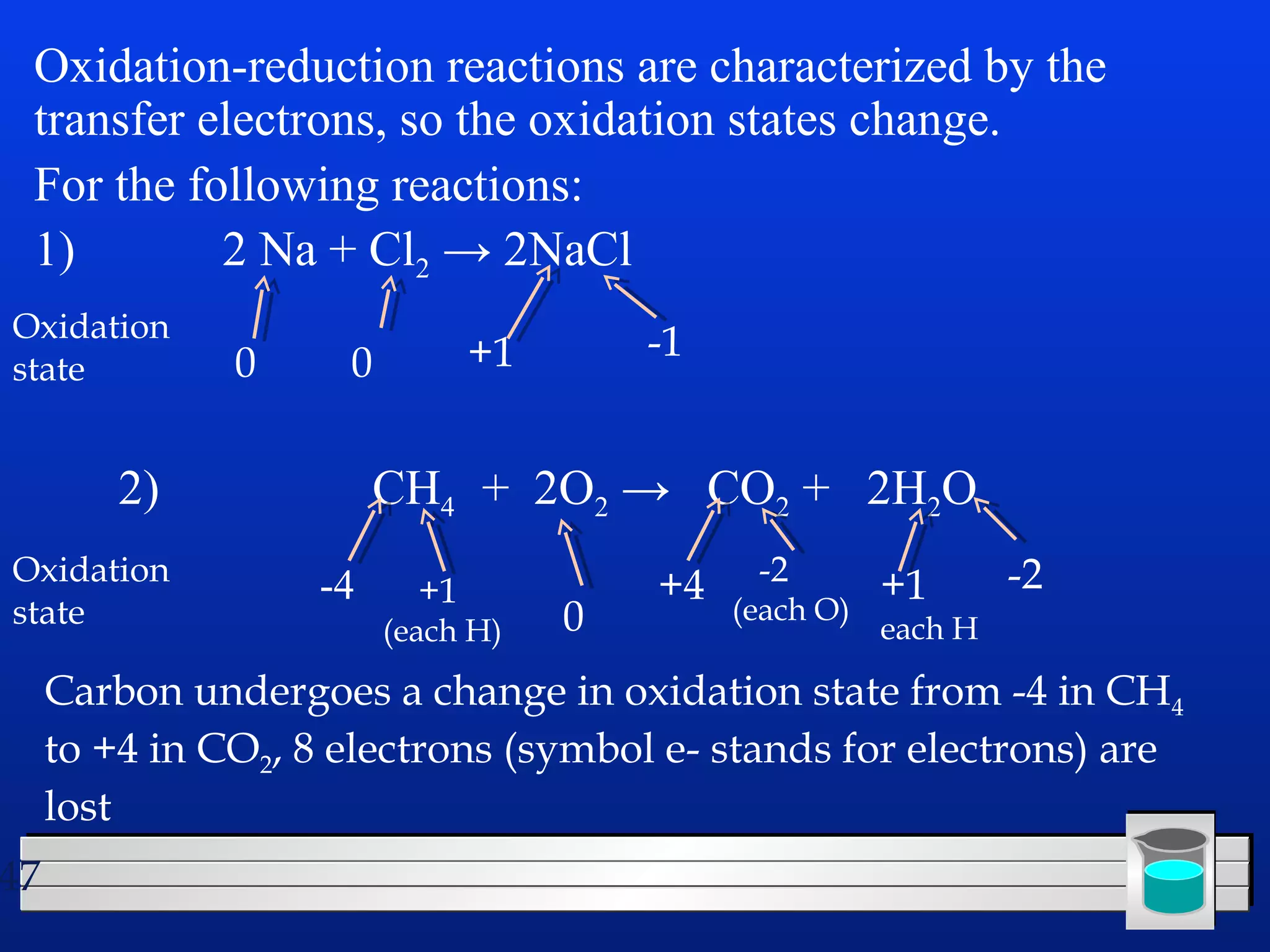
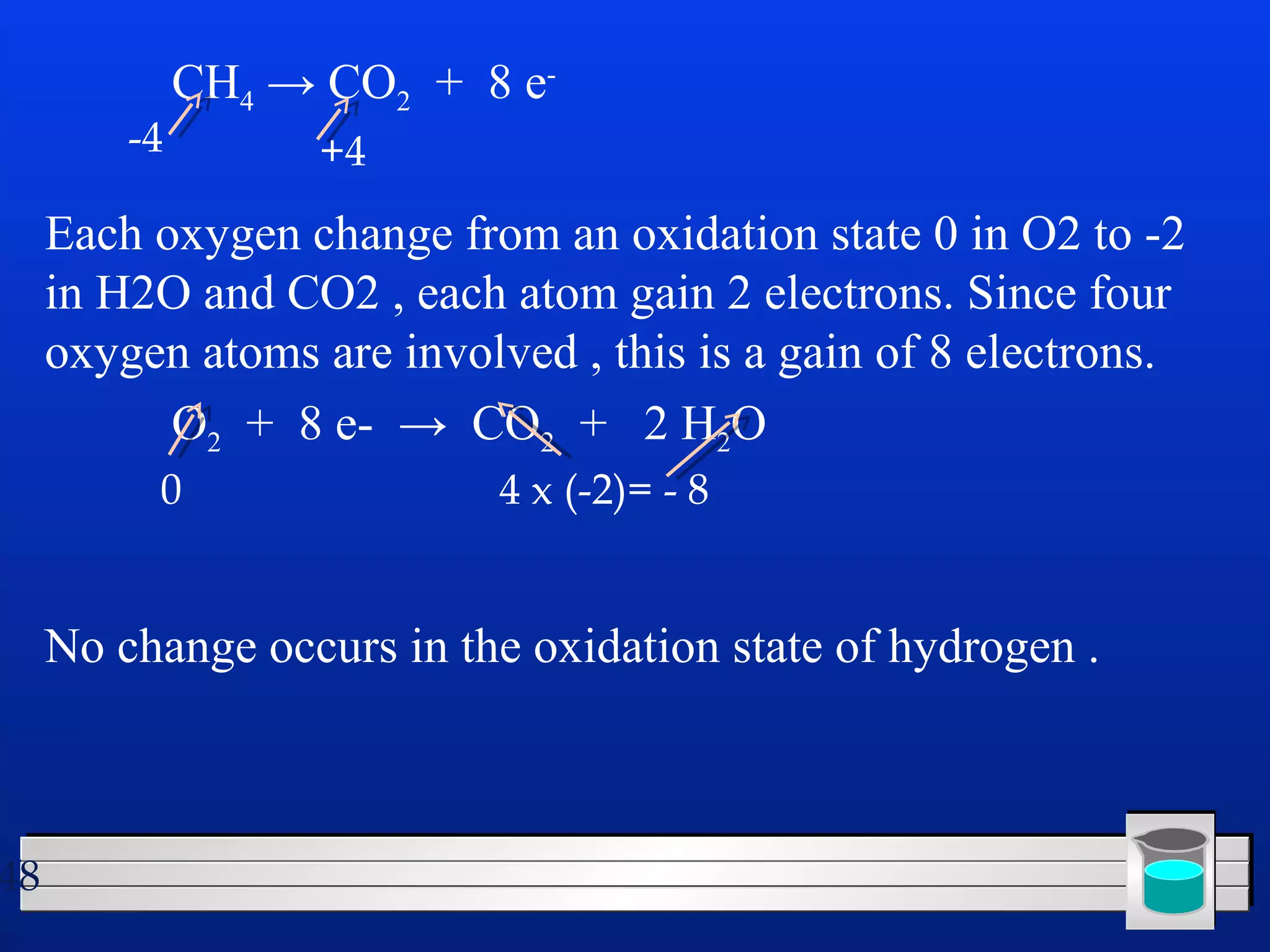
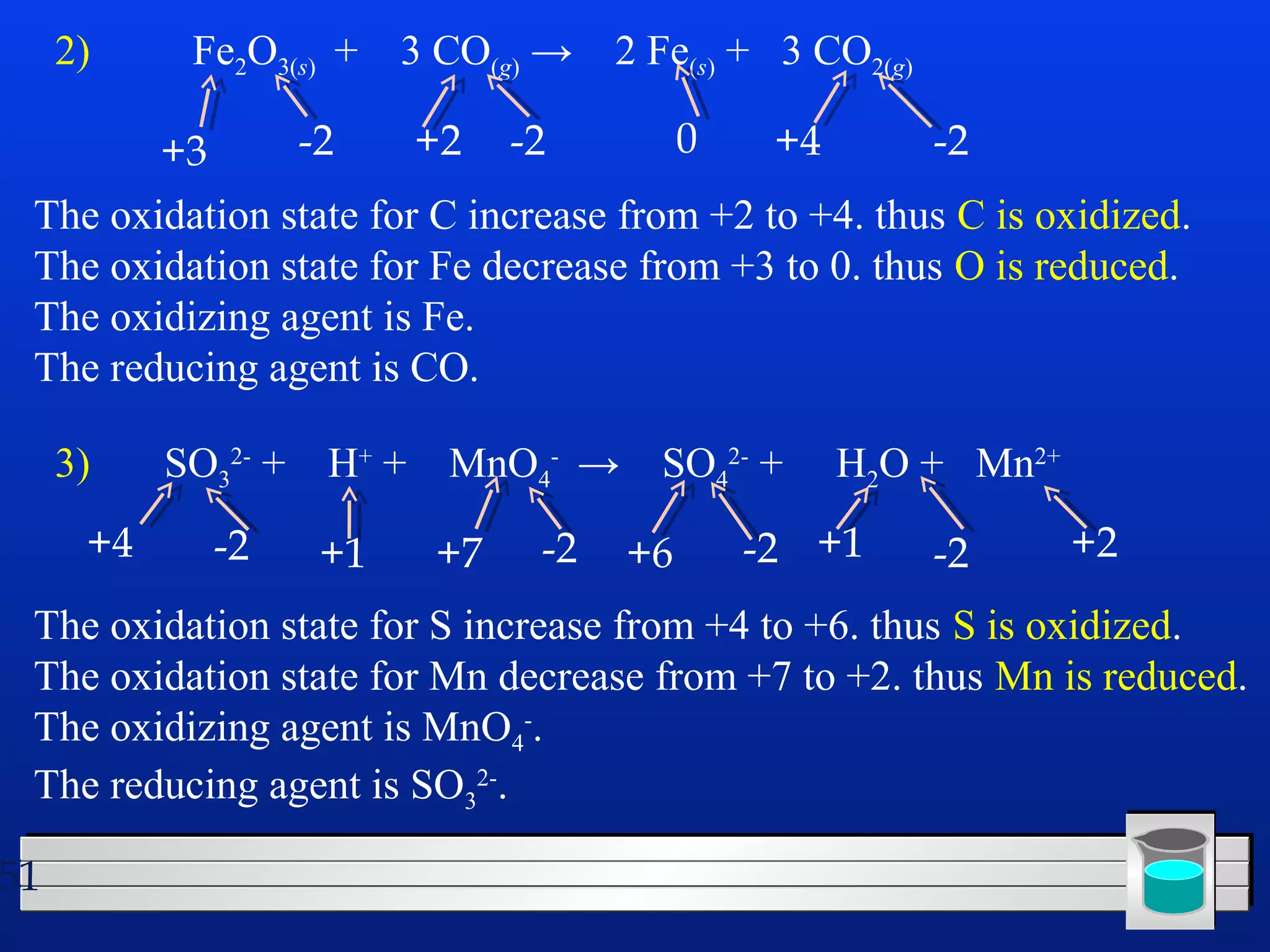
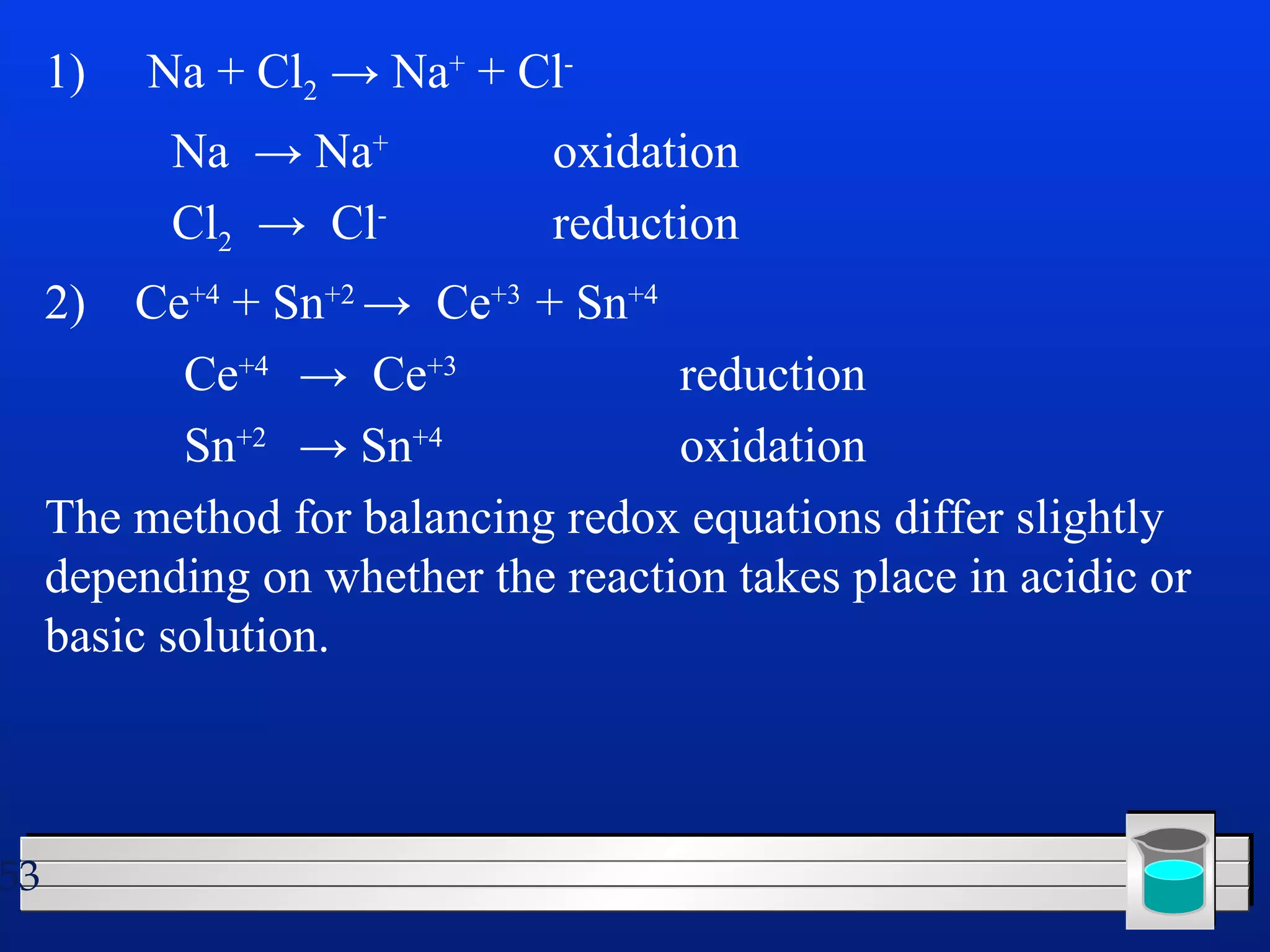
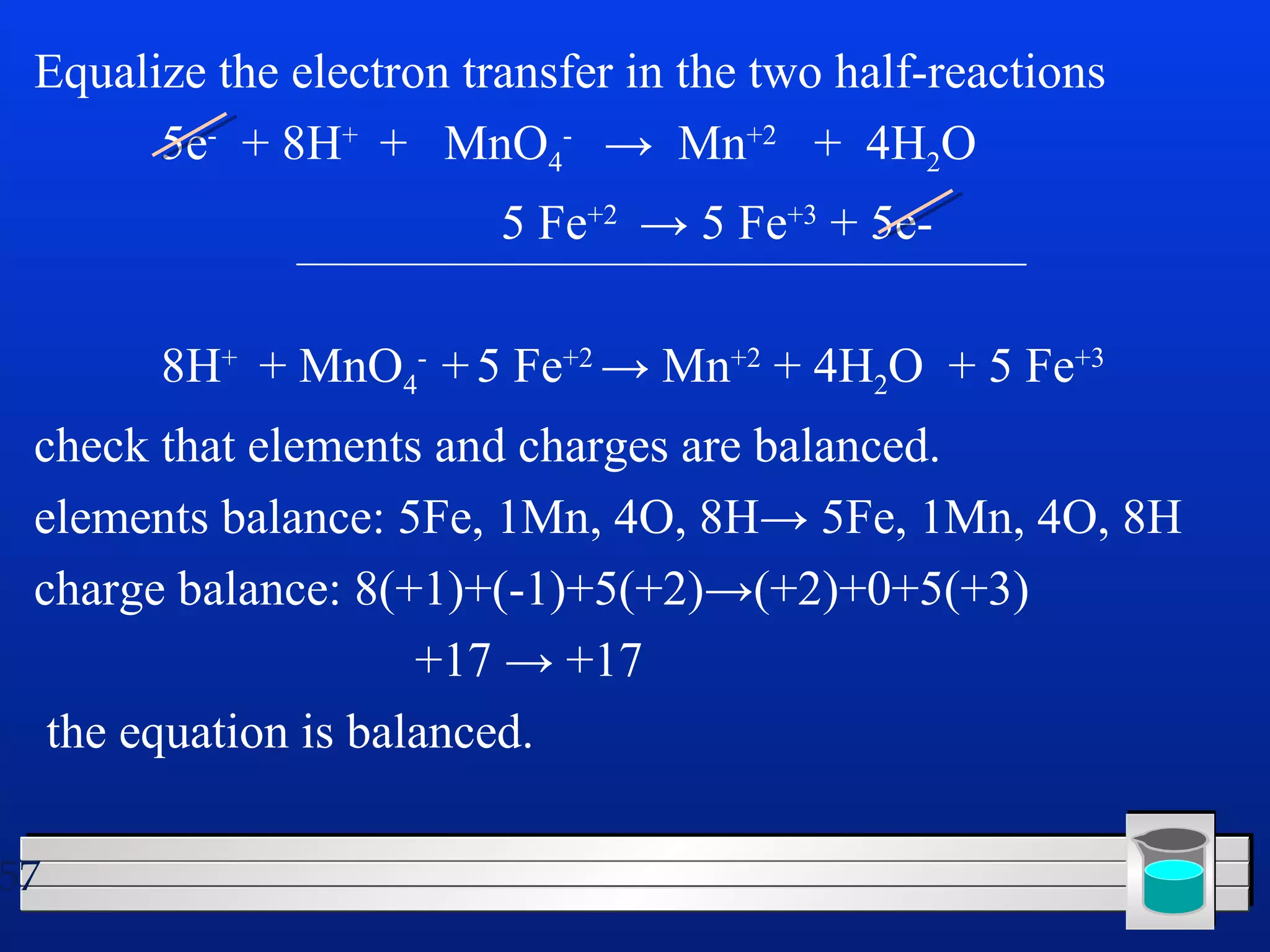
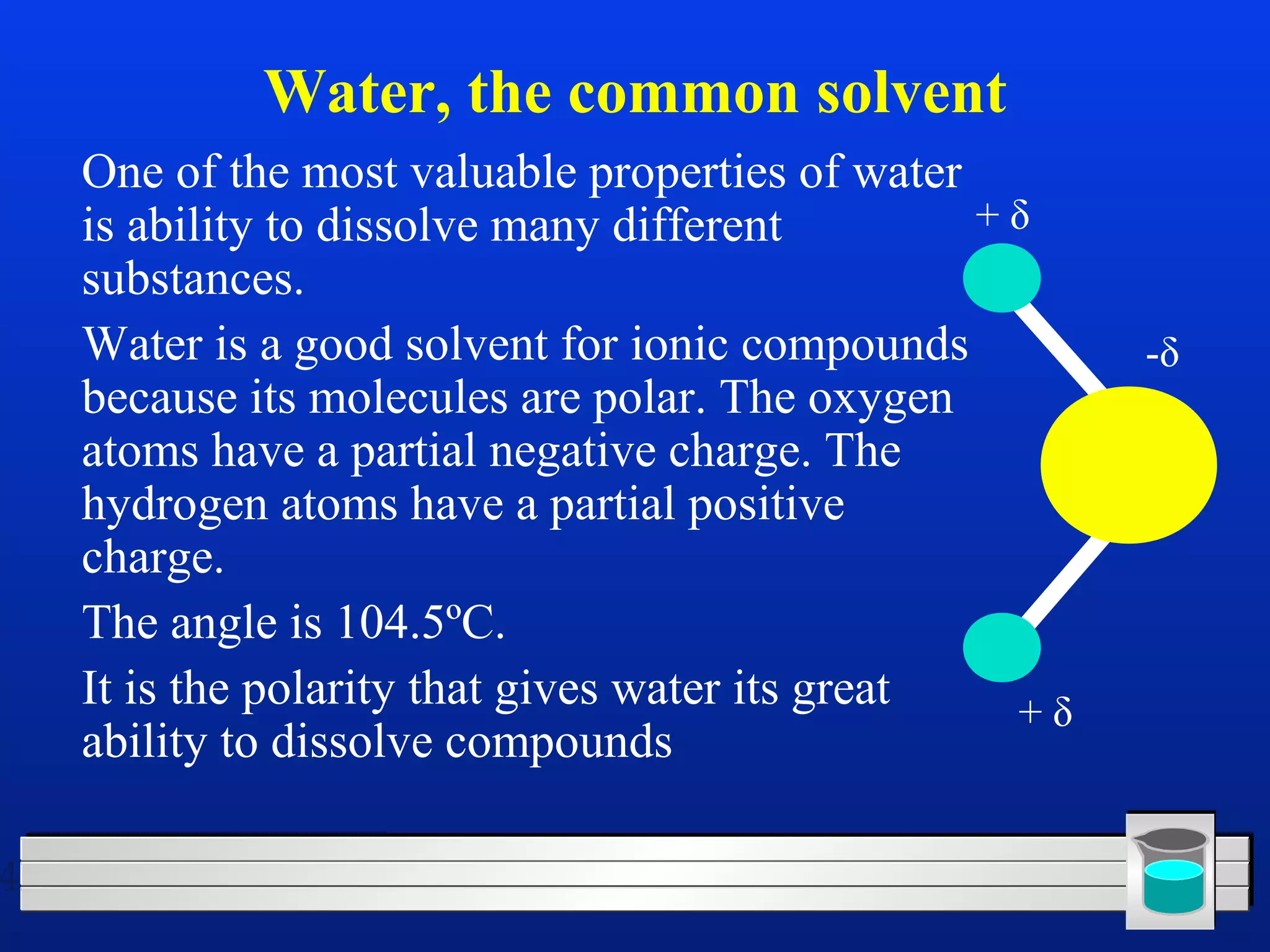
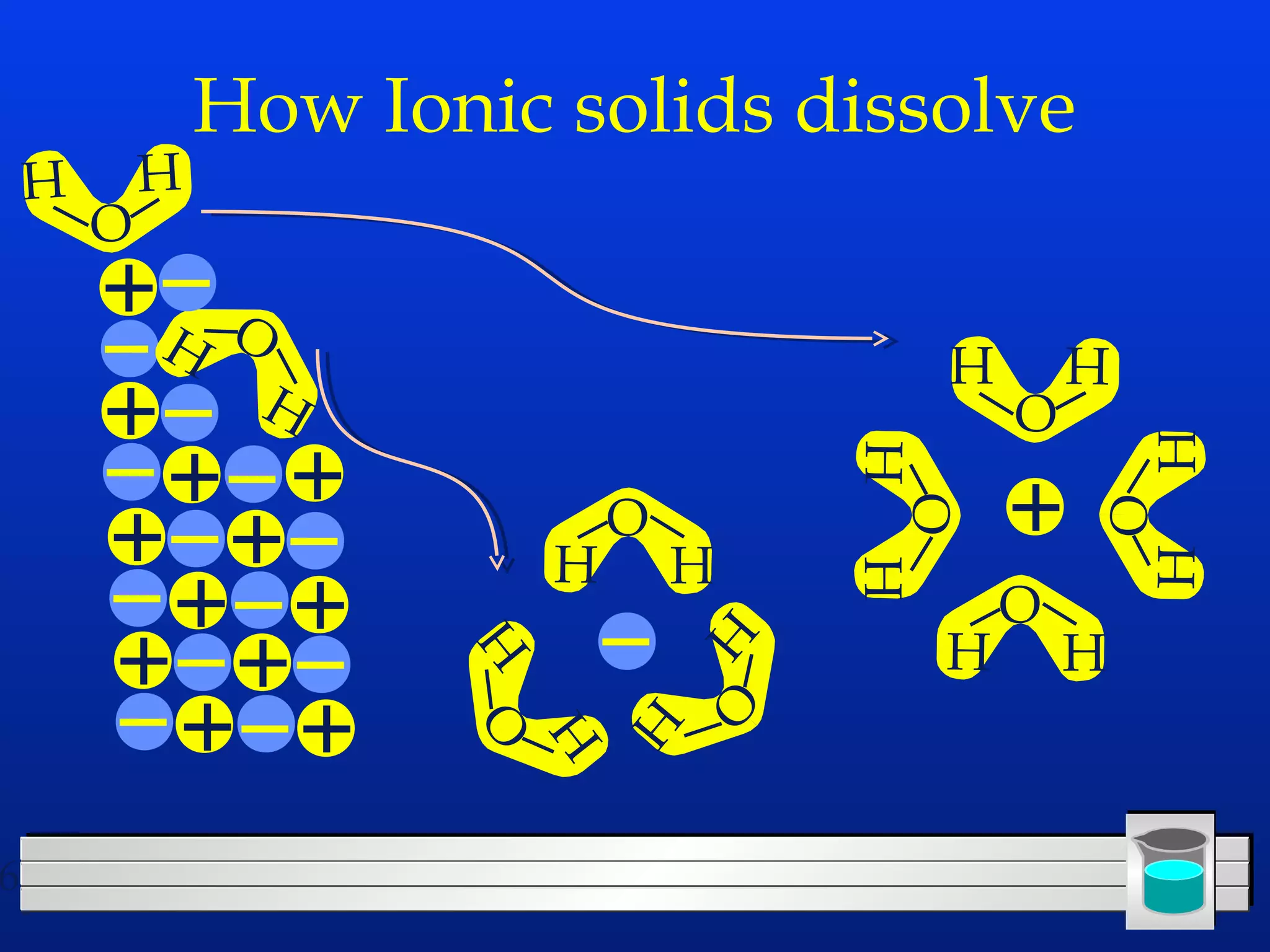
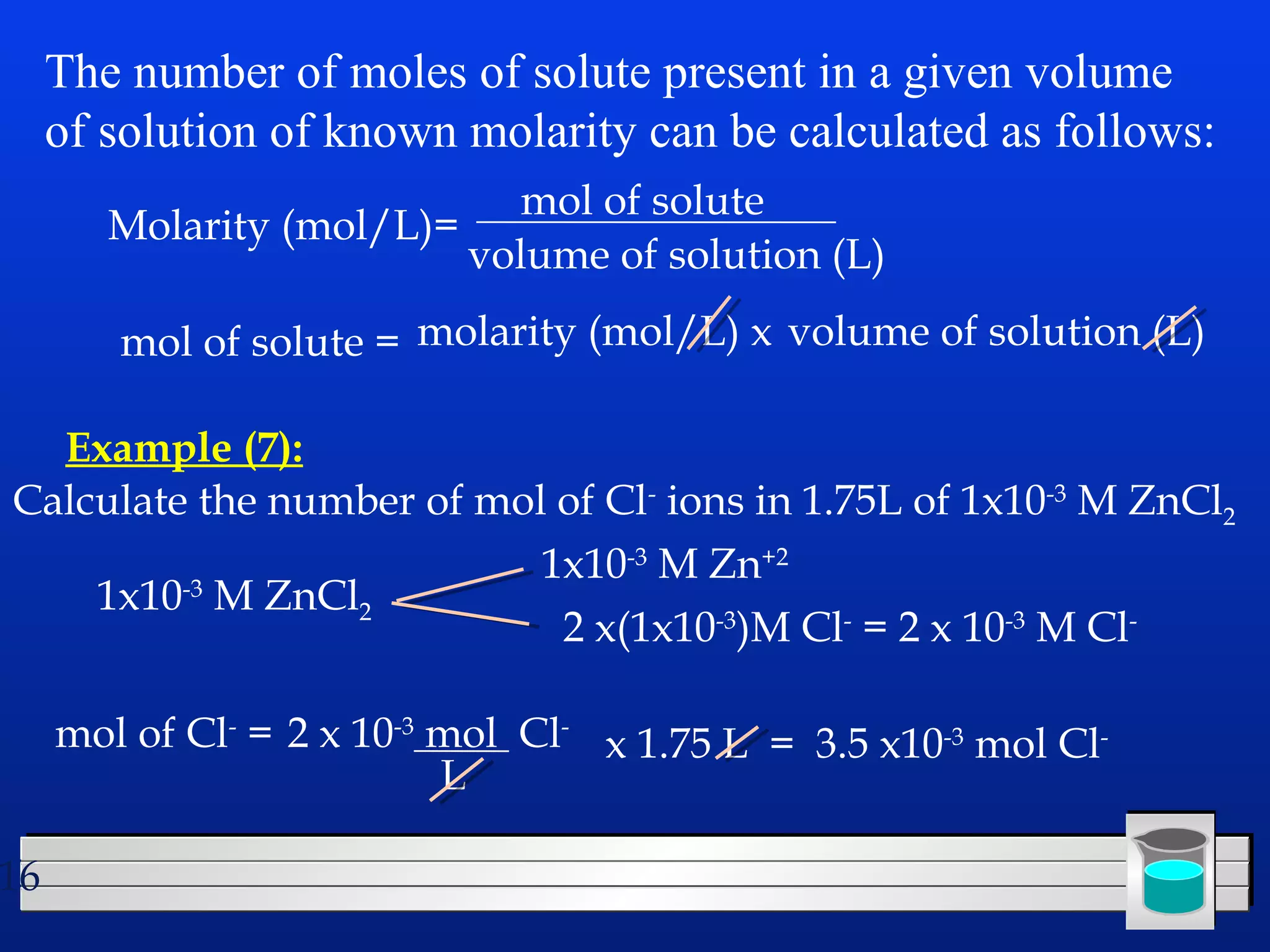
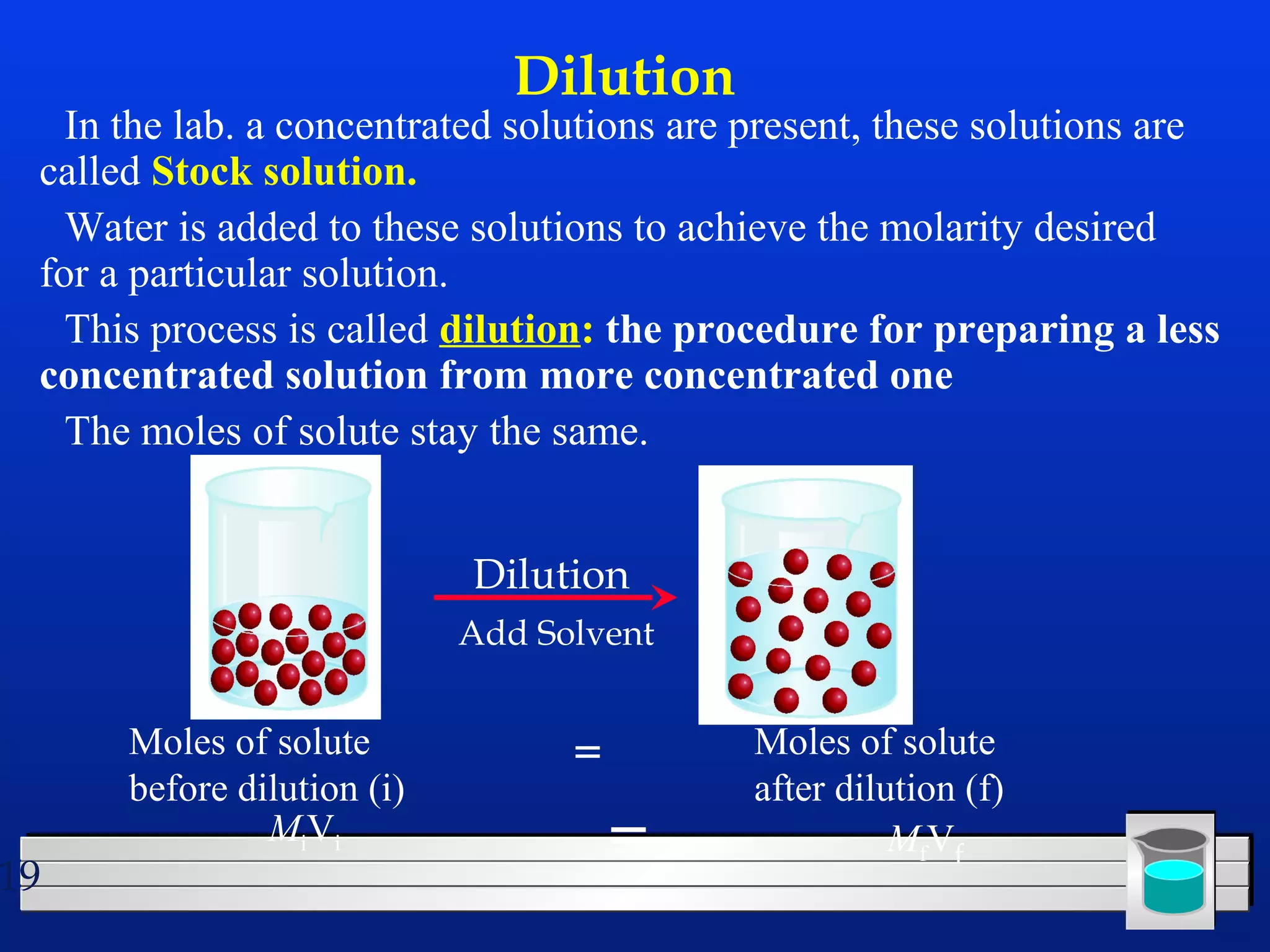
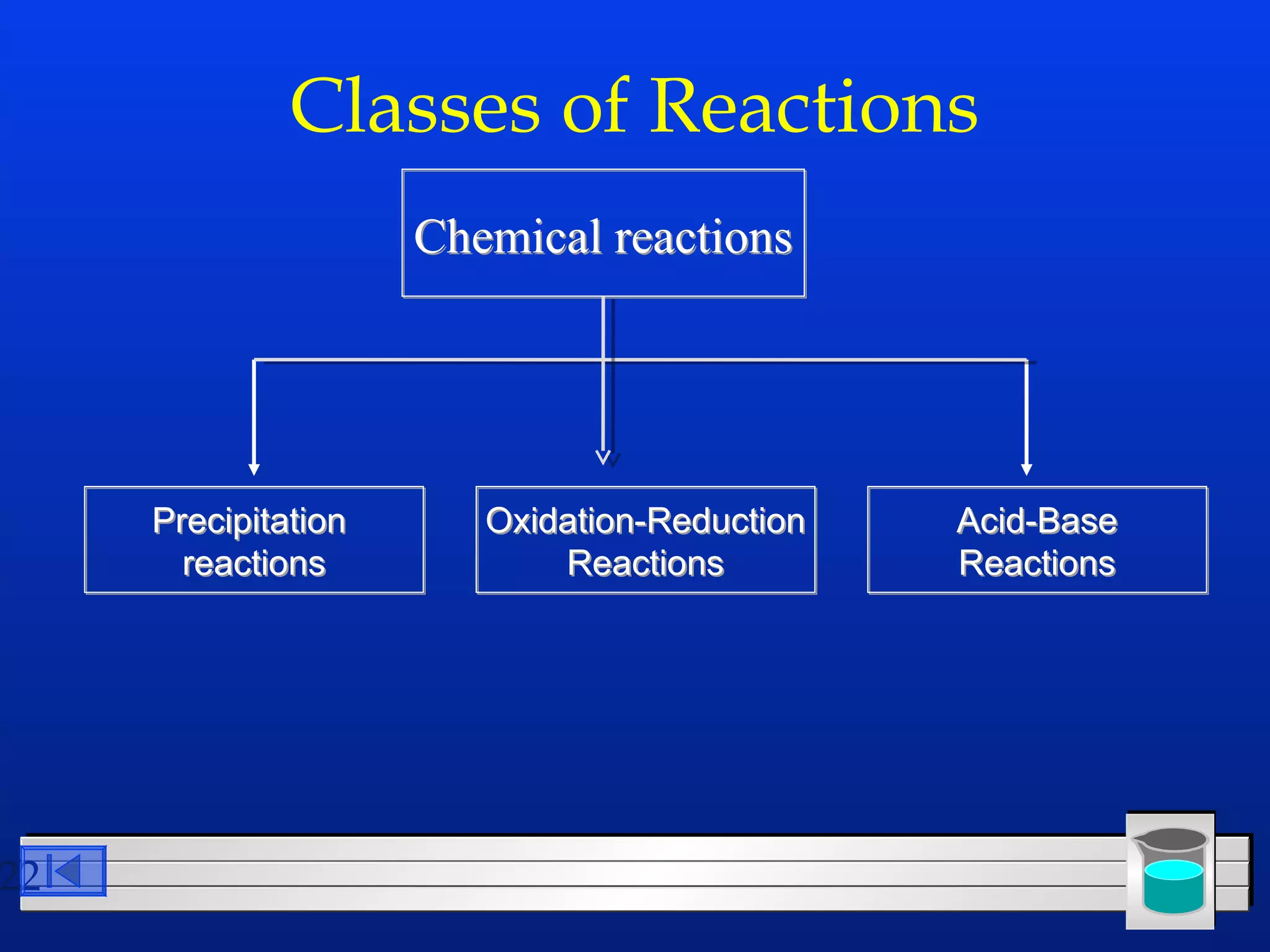
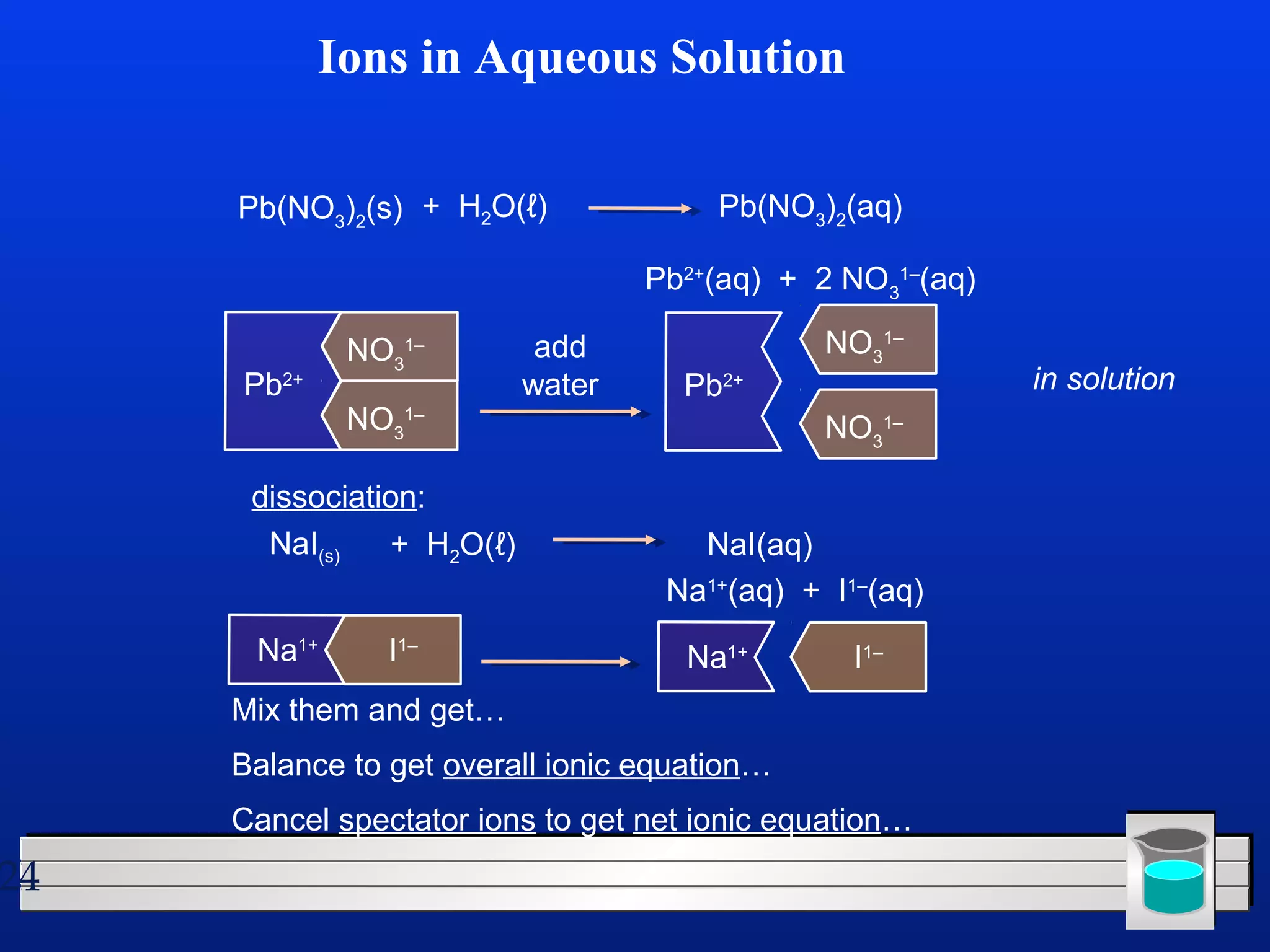
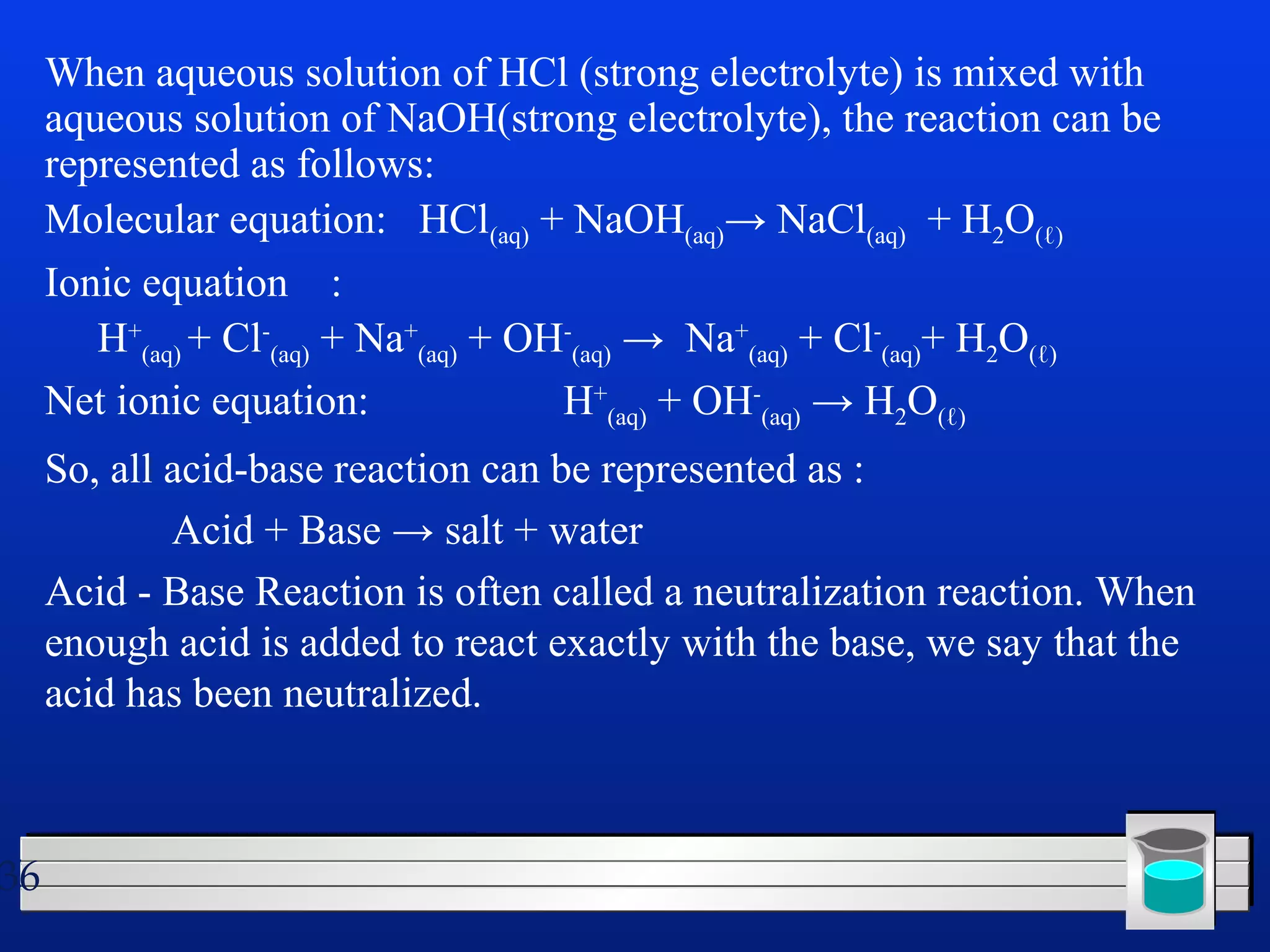
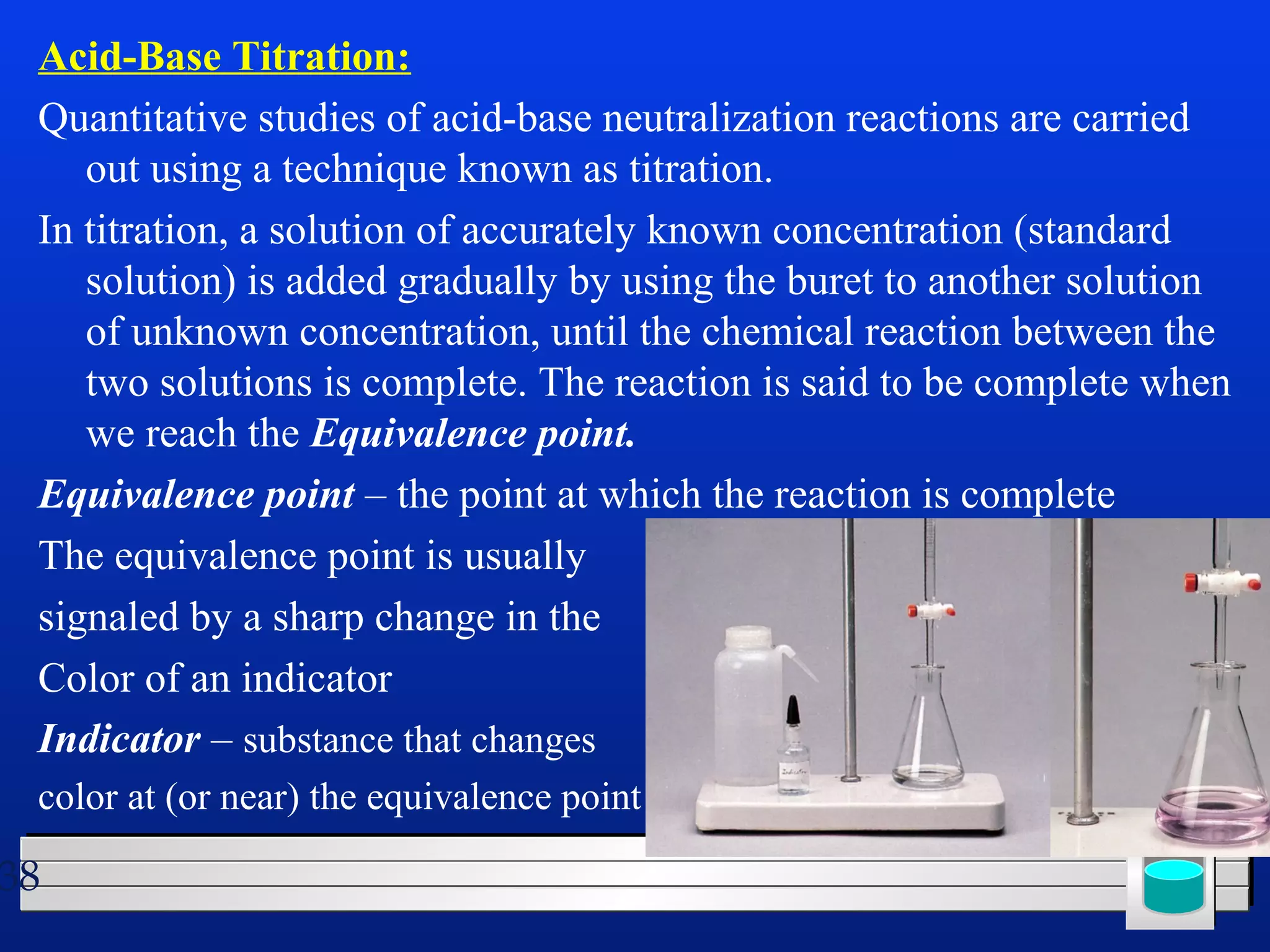
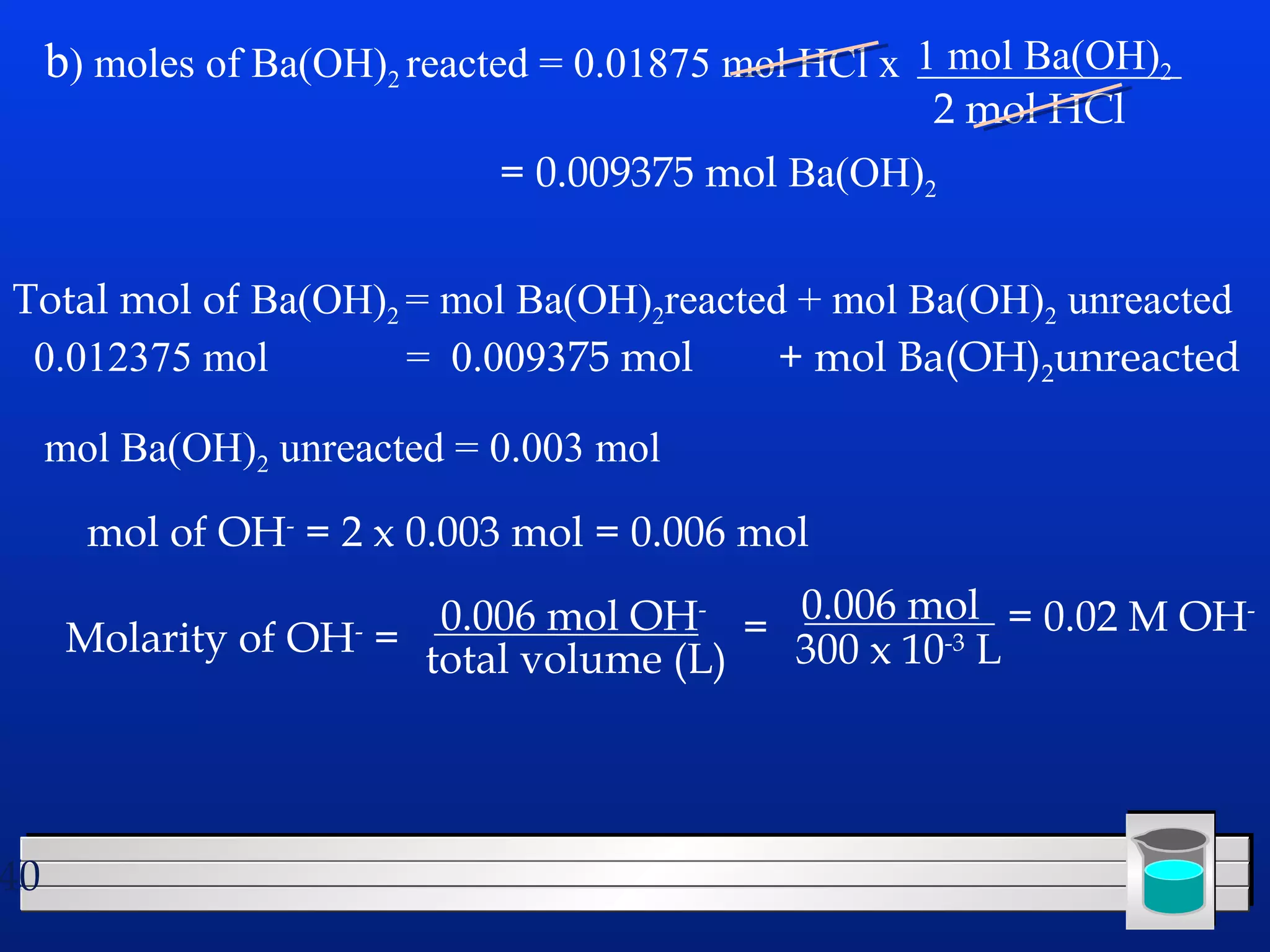
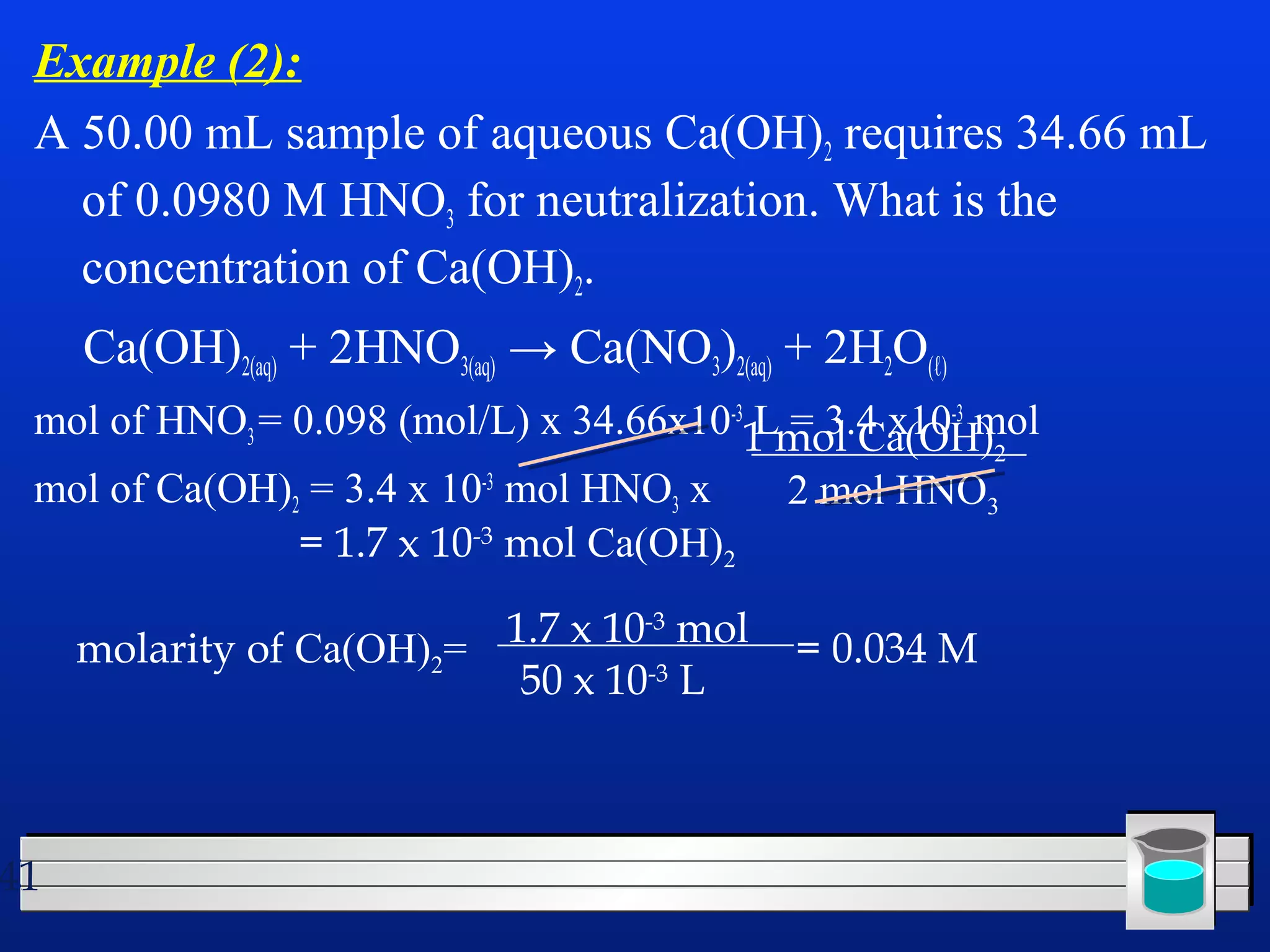
Many chemical reactions occur in water. Water is a polar solvent that can dissolve ionic compounds via hydration. When an ionic compound dissolves in water, it separates into its constituent ions which are surrounded by water molecules. The concentration of a solution is expressed as molarity, which is the number of moles of solute per liter of solution. Solutions can be prepared by accurately weighing out and dissolving the solute in a volumetric flask and diluting the solution as needed.























![Example:
Assign the oxidation states to each element in the following
compounds.
CO2 : O is -2, so -2 x 2 + C = 0 C = +4
NO3
46
- : O is -2, so (3 x -2)+ N = -1 N = +5
H2SO4 : H is +1, O is -2, so [2x(+1)]+[(4x(-2)]+S =0
S = +6
FeO: O is -2, so [3x(-2)]+ 2xFe = 0 Fe = +3
23 CrO-2 : 2xCr +[7x(-2)]= -2 Cr = +6
27](https://image.slidesharecdn.com/4reactioninaq-141127005934-conversion-gate01/75/4-reaction-in-aq-solution-46-2048.jpg)