This document discusses chemical equilibrium. It begins by explaining that many chemical reactions do not go to completion, but rather reach a state of dynamic equilibrium where the rates of the forward and reverse reactions are equal. This equilibrium state occurs when the concentrations of reactants and products remain constant over time.
It then introduces the equilibrium constant expression (K), which relates the concentrations or pressures of products and reactants at equilibrium. The value of K is unique to a particular chemical reaction at a given temperature. Examples are provided to demonstrate how K is calculated from experimental equilibrium concentrations. The summary concludes by noting that K can be expressed in terms of either molar concentrations (Kc) or partial pressures (Kp), and the relationship between these two expressions





![7
For the reverse reaction we have,
C + D A + B Rr = kr [C][D]
We know at equil that Rf = Rr ;therefore, we can set these two
expressions as equal
kf [A][B] = kr [C][D]
Rearrange to put constants on one side we get
]
][
[
]
][
[
B
A
D
C
k
k
K
r
f
If we assume these reactions are elementary rxns (based on
collisions), we can write the rate laws directly from the
reaction:
A + B C + D Rf = kf [A][B]](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-6-320.jpg)
![8
Constant divided by constant just call a new constant K. This ratio is
given a special name and symbol called equilibrium constant K
relating to the equilibrium condition at a certain temperature (temp
dependent) for a particular reaction relating conc of each component.
This is basically a comparison between forward and reverse reaction
rates. At equilibrium, the ratio of conc of species must satisfy K.
]
][
[
]
][
[
B
A
D
C
k
k
K
r
f
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-7-320.jpg)

![10
The Equilibrium Constant
• The equilibrium-constant expression for a reaction is
obtained by multiplying the equil concentrations ( or partial
pressures) of products, dividing by the equil concentrations (or
partial pressures) of reactants, and raising each concentration to
a power equal to its coefficient in the balanced chemical
equation.
dD
cC
bB
aA
b
a
d
c
B
A
D
C
K
]
[
]
[
]
[
]
[
b
B
a
A
d
D
c
C
p
P
P
P
P
K
)
(
)
(
)
(
)
(
c
The molar concentration of a substance is denoted by writing its formula in
square brackets for aq solutions. For gases can put Pa - atm. As long as use M
or atm, K is unitless; liquids and solids = 1; setup same for all K’s (Ka, Kb, etc.)
Temp dependent; any changes, ratio will still equal K when equil established](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-9-320.jpg)


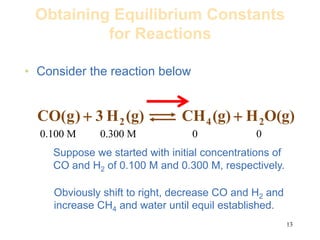
![14
– When the system finally settled into equilibrium we
determined the equilibrium concentrations to be as
follows.
Reactants Products
[CO]eq = 0.0613 M
[H2] eq = 0.1839 M
[CH4] eq = 0.0387 M
[H2O] eq = 0.0387 M
Obtaining Equilibrium Constants
for Reactions
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-13-320.jpg)
![15
– If we substitute the equilibrium concentrations,
we obtain:
93
.
3
)
1839
.
0
)(
0613
.
0
(
)
0387
.
0
)(
0387
.
0
(
3
c
K
Obtaining Equilibrium Constants
for Reactions
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
eq
eq
eq
eq
c
H
CO
O
H
CH
K 3
2
2
4
]
[
]
[
]
[
]
[
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-14-320.jpg)

![17
As an example, let’s repeat the previous
experiment, only this time starting with initial
concentrations of products (note: if only products
to start shift left until equil established):
[CH4]initial = 0.2000 M and [H2O]initial = 0.2000 M
Obviously shift to left decrease CH4 and H2O and
increase CO and H2 until equil established.
Obtaining Equilibrium Constants
for Reactions
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
0 0 0.2000 M 0.2000 M](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-16-320.jpg)
![18
– We find that these initial concentrations result in
the following equilibrium concentrations.
Reactants Products
[CO] eq = 0.0990 M
[H2] eq = 0.2970 M
[CH4] eq = 0.1010 M
[H2O] eq = 0.1010 M
Obtaining Equilibrium Constants
for Reactions
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-17-320.jpg)
![19
– Substituting these values into the equilibrium-
constant expression, we obtain the same result.
93
.
3
)
2970
.
0
)(
0990
.
0
(
)
1010
.
0
)(
1010
.
0
(
3
c
K
– Whether we start with reactants or products at any
initial conc, the system establishes the same ratio
at same temperature.
Obtaining Equilibrium Constants
for Reactions
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
eq
eq
eq
eq
c
H
CO
O
H
CH
K 3
2
2
4
]
[
]
[
]
[
]
[
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-18-320.jpg)



![23
The Equilibrium Constant, Kp
– Consider the reaction below.
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
– The equilibrium-constant expression in terms of partial
pressures becomes (same way but partial pressures instead
of M):
3
)
( 2
2
4
H
CO
O
H
CH
p
K
P
P
P
P
3
2
2
4
]
[H
]
[
O]
[
]
[
CO
H
CH
Kc ](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-22-320.jpg)




![28
A Problem to Consider
• Applying Stoichiometry to an Equilibrium Mixture
(basic setup for future problems).
– What is the composition of the equilibrium mixture
if it contains 0.080 mol NH3 at equilibrium?
– Suppose we place 1.000 mol N2 and 3.000 mol H2
in a reaction vessel at 450 oC and 10.0
atmospheres of pressure. The reaction is
(g)
2NH
)
g
(
H
3
)
g
(
N 3
2
2
3
2
2
2
3
c
]
H
][
N
[
]
NH
[
K ](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-27-320.jpg)







![36
Heterogeneous Equilibria
• Consider the reaction below.
(g)
H
CO(g)
)
(
)
( 2
2
l
O
H
s
C
2
2 ]
][
[
H
CO
p
c
P
P
K
H
CO
K
HW 17
code: mike](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-35-320.jpg)

![38
Predicting the Direction of Reaction
• For the general reaction
dD
cC
bB
aA
the Qc expresssion would be (i=initial):
b
a
d
c
c
]
B
[
]
A
[
]
D
[
]
C
[
Q
i
i
i
i
b
a
d
c
c
B
A
D
C
K
eq
eq
eq
eq
]
[
]
[
]
[
]
[
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-37-320.jpg)
![39
Predicting the Direction of Reaction
• For the general reaction
dD
cC
bB
aA
– If Qc = Kc, then the reaction is at equilibrium.
– If Qc > Kc, the reaction will shift left toward reactants until equil
reached.
– If Qc < Kc, the reaction will shift right toward products until equil
reached.
b
a
d
c
c
]
B
[
]
A
[
]
D
[
]
C
[
Q
i
i
i
i
b
a
d
c
c
]
B
[
]
A
[
]
D
[
]
C
[
Q
i
i
i
i
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-38-320.jpg)

![41
A Problem to Consider
First, calculate concentrations from moles
of substances.
(g)
2NH
)
g
(
H
3
)
g
(
N 3
2
2
3
2
2
2
3
]
[
]
[
]
[
i
i
i
c
H
N
NH
Q
Next, plug into Q expression](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-40-320.jpg)




![46
Calculating Equilibrium
Concentrations
– First, calculate concentrations from moles of substances
then plug into K expression.
O(g)
H
(g)
CH
(g)
H
3
)
( 2
4
2
g
CO
eq
eq
eq
eq
c
H
CO
O
H
CH
K 3
2
2
4
]
[
]
[
]
[
]
[
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-45-320.jpg)

H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-46-320.jpg)
![48
Calculating Equilibrium
Concentrations
– You can now solve for [CH4].
M
CH 059
.
0
)
020
.
0
(
)
10
.
0
)(
30
.
0
)(
92
.
3
(
]
[
3
4
– The concentration of CH4 in the equil mixture is
0.059 mol/L.
??
0.30 M 0.10 M 0.020 M
O(g)
H
(g)
CH
(g)
H
3
)
g
(
CO 2
4
2
](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-47-320.jpg)




![53
Calculating Equilibrium
Concentrations
– Let x be the moles per liter of product formed.
Initial 0.0200 0.0200 0 0
Change
Equil
– The equilibrium-constant expression is:
]
O
H
][
CO
[
]
H
][
CO
[
K
2
2
2
c
(g)
H
(g)
CO
)
g
(
O
H
)
g
(
CO 2
2
2
- - + +
x x x x
0.0200-x 0.0200-x x x](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-52-320.jpg)
![54
– Solving for x.
Initial 0.0200 0.0200 0 0
Change -x -x +x +x
Equilibrium0.0200-x 0.0200-x x x
– Substituting the values for equilibrium concentrations, we
get:
)
x
0200
.
0
)(
x
0200
.
0
(
)
x
)(
x
(
58
.
0
(g)
H
(g)
CO
)
g
(
O
H
)
g
(
CO 2
2
2
]
O
H
][
CO
[
]
H
][
CO
[
K
2
2
2
c ](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-53-320.jpg)


![57
Calculating Equilibrium
Concentrations
– Solving for equilibrium concentrations.
Initial 0.0200 0.0200 0 0
Change -x -x +x +x
Equilibrium0.0200-x 0.0200-x x x
– If you substitute for x in the last line of the table you obtain the
following equilibrium concentrations. If plug into Keq, should
equal K or close to it because of sign fig for a check
(g)
H
(g)
CO
)
g
(
O
H
)
g
(
CO 2
2
2
HW 19
[CO]eq = 0.0200 – x = 0.0200 – 0.0086 = 0.0114 M
[H2O]eq = 0.0200 – x = 0.0200 – 0.0086 = 0.0114 M
[CO2]eq = x = 0.0086 M
[H2]eq = x = 0.0086 M
code: katie](https://image.slidesharecdn.com/chapter31-230817140206-f20c584c/85/chapter31-ppt-56-320.jpg)























