This document provides an introduction to fundamentals of electrochemistry, including:
1. Redox reactions involve the transfer of electrons between chemical species and can be monitored by measuring electric current.
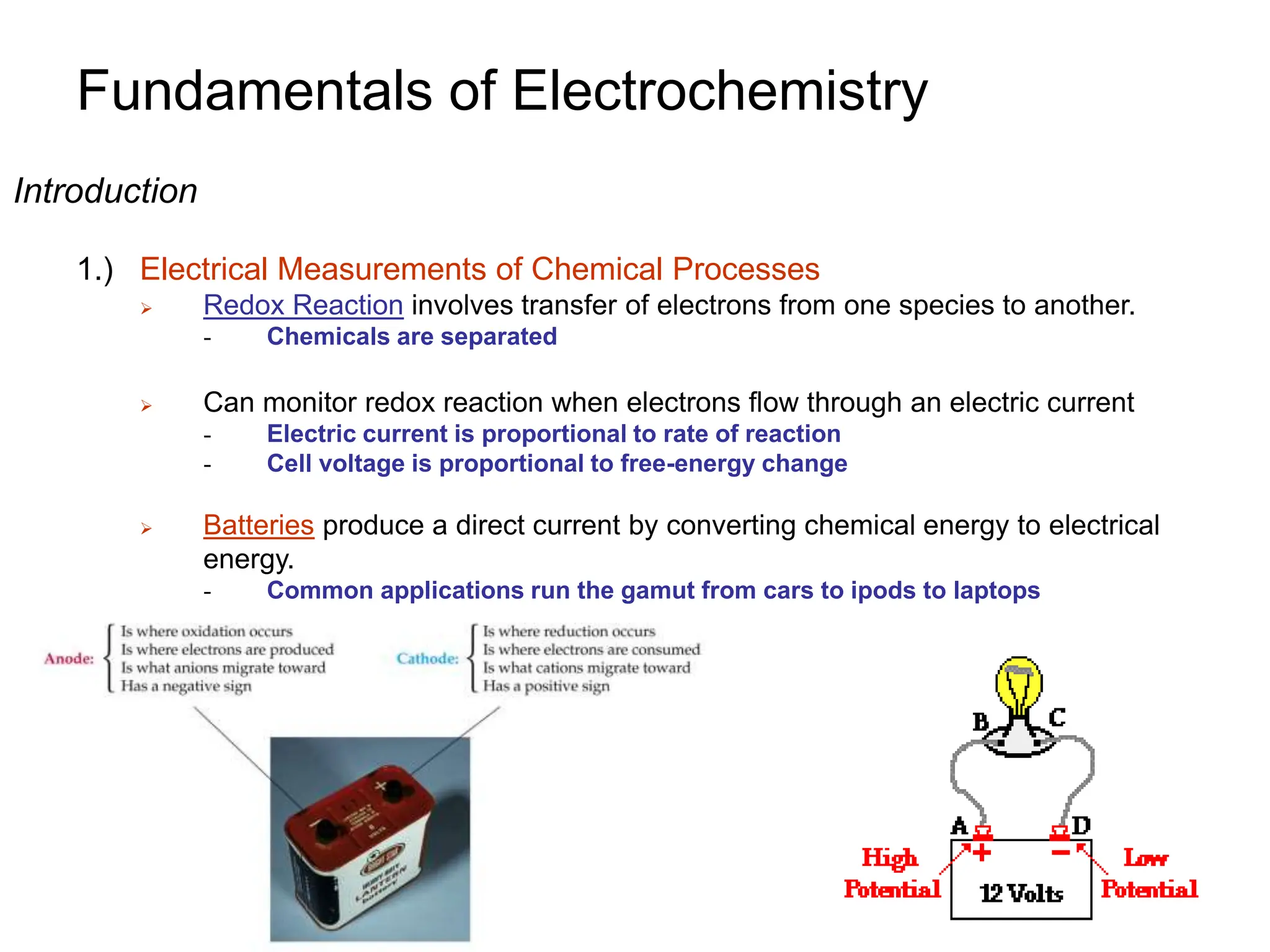
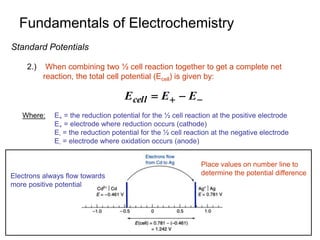
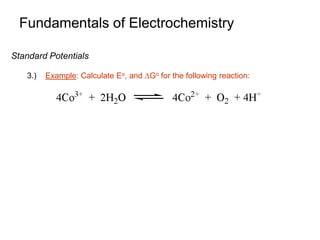
2. A redox titration uses the transfer of electrons between analyte and titrant for analytical purposes. Reduction occurs when a substance gains electrons and oxidation occurs when a substance loses electrons.
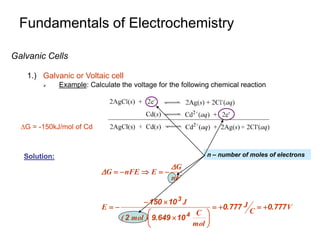
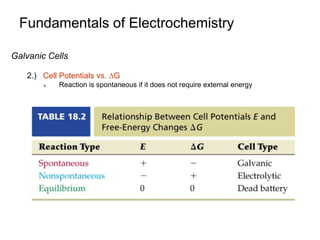
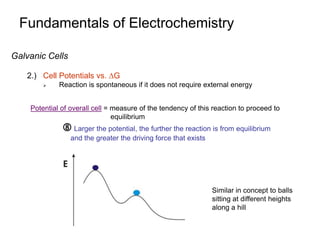
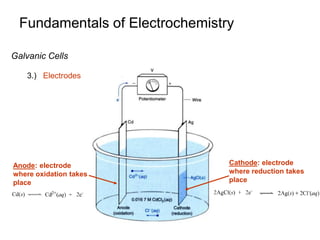
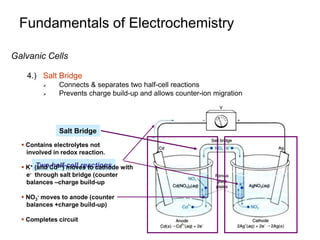
3. Galvanic cells use spontaneous redox reactions to generate electricity by separating the half-cell reactions and allowing electrons to flow through an external circuit.
![Fundamentals of Electrochemistry
Nernst Equation
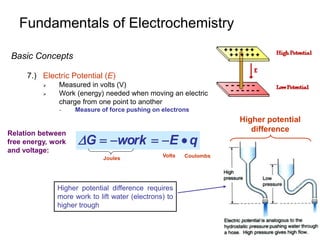
1.) Reduction Potential under Non-standard Conditions
E determined using Nernst Equation
Concentrations not-equal to 1M
aA + ne- bB Eo
For the given reaction:
The ½ cell reduction potential is given by:
a
b
o
a
A
b
B
o
]
A
[
]
B
[
log
n
V
E
E
A
A
ln
nF
RT
E
E
0.05916
Where: E = actual ½ cell reduction potential
Eo = standard ½ cell reduction potential
n = number of electrons in reaction
T = temperature (K)
R = ideal gas law constant (8.314J/(K-mol)
F = Faraday’s constant (9.649x104 C/mol)
A = activity of A or B
at 25oC](https://image.slidesharecdn.com/chapter-14-231009065519-fdc16ef3/85/chapter-14-ppt-19-320.jpg)
![Fundamentals of Electrochemistry
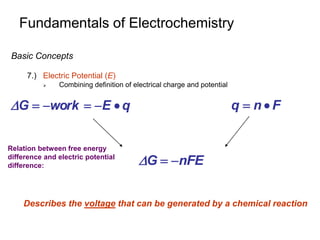
Eo and the Equilibrium Constant
1.) A Galvanic Cell Produces Electricity because the Cell Reaction is
NOT at Equilibrium
Concentration in two cells change with current
Concentration will continue to change until Equilibrium is reached
- E = 0V at equilibrium
- Battery is “dead”
d
b
o
a
c
o
cell
]
D
[
]
B
[
log
n
.
E
]
A
[
]
C
[
log
n
.
E
E
E
E
05916
0
05916
0
aA + ne- cC E+
o
dD + ne- bB E-
o
Consider the following ½ cell reactions:
Cell potential in terms of Nernst Equation is:
b
a
d
c
o
o
cell
]
B
[
]
A
[
]
D
[
]
C
[
log
n
.
)
E
E
(
E
05916
0
Simplify:](https://image.slidesharecdn.com/chapter-14-231009065519-fdc16ef3/85/chapter-14-ppt-21-320.jpg)
![b
a
d
c
o
cell
]
B
[
]
A
[
]
D
[
]
C
[
log
n
.
E
E
05916
0
Fundamentals of Electrochemistry
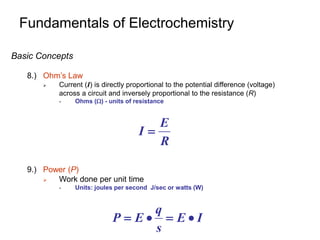
Eo and the Equilibrium Constant
1.) A Galvanic Cell Produces Electricity because the Cell Reaction is
NOT at Equilibrium
Since Eo=E+
o- E-
o:
At equilibrium Ecell =0:
K
log
n
.
Eo 05916
0
Definition of
equilibrium constant
05916
0
10 .
nEo
K
at 25oC
at 25oC](https://image.slidesharecdn.com/chapter-14-231009065519-fdc16ef3/85/chapter-14-ppt-22-320.jpg)
![Fundamentals of Electrochemistry
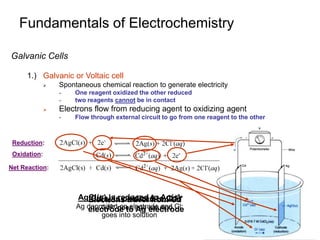
Biochemists Use Eo´
1.) Redox Potentials Containing Acids or Bases are pH Dependent
Standard potential all concentrations = 1 M
pH=0 for [H+] = 1M
2.) pH Inside of a Plant or Animal Cell is ~ 7
Standard potentials at pH =0 not appropriate for biological systems
- Reduction or oxidation strength may be reversed at pH 0 compared to pH 7
Metabolic Pathways](https://image.slidesharecdn.com/chapter-14-231009065519-fdc16ef3/85/chapter-14-ppt-26-320.jpg)
![Fundamentals of Electrochemistry
Biochemists Use Eo´
3.) Formal Potential
Reduction potential that applies
under a specified set of
conditions
Formal potential at pH 7 is Eo´
b
a
d
c
o
cell
]
B
[
]
A
[
]
D
[
]
C
[
log
n
.
E
E
05916
0
Need to express concentrations as
function of Ka and [H+].
Cannot use formal concentrations!
Eo´ (V)](https://image.slidesharecdn.com/chapter-14-231009065519-fdc16ef3/85/chapter-14-ppt-27-320.jpg)