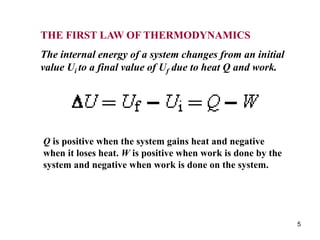
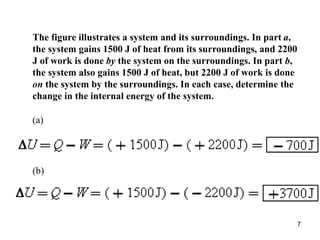
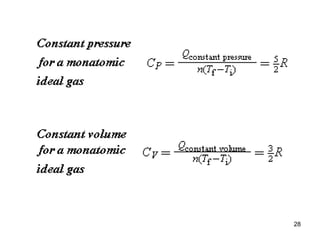
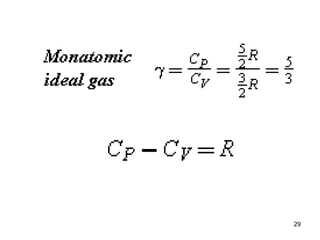
1) Thermodynamics is the branch of physics that deals with heat and work. The first law of thermodynamics states that the change in internal energy of a system equals the heat added to the system minus the work done by the system.
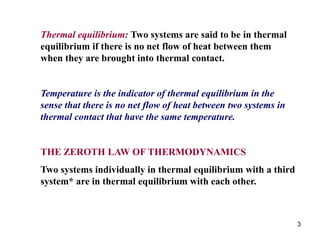
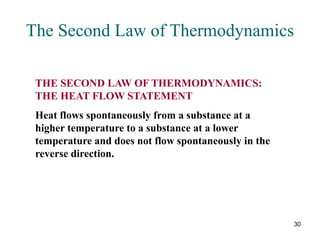
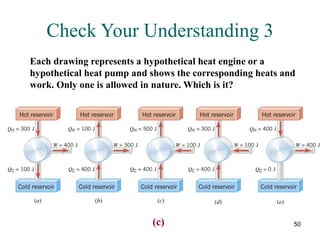
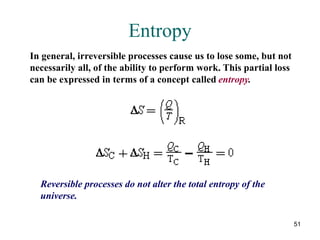
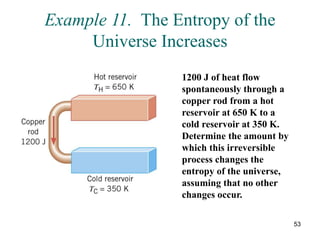
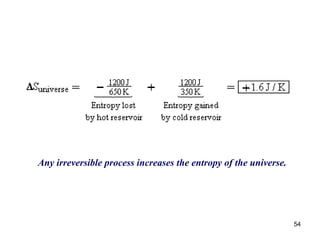
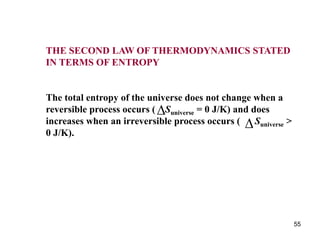
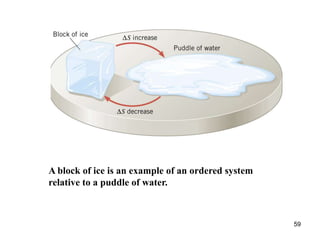
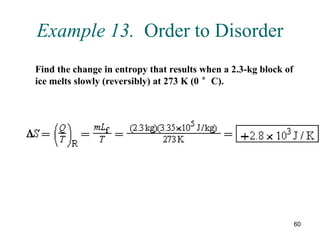
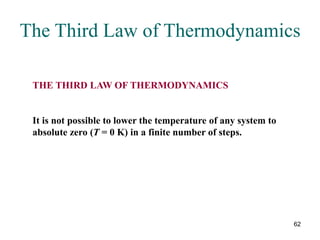
2) The second law of thermodynamics states that heat cannot spontaneously flow from a cooler body to a hotter body. All real-world processes are irreversible and cause the entropy of the universe to increase.
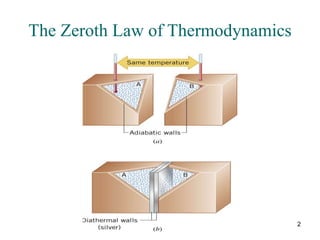
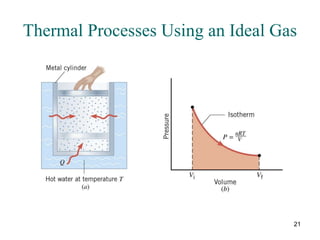
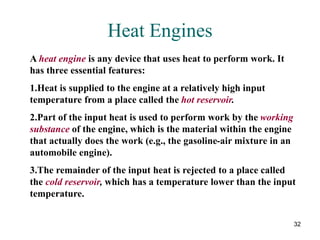
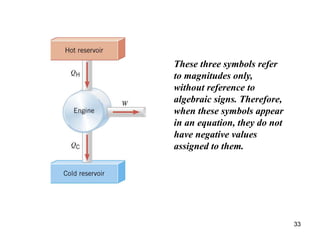
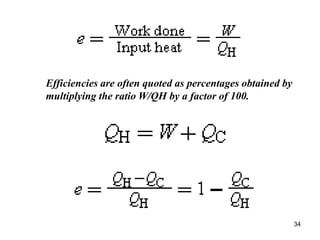
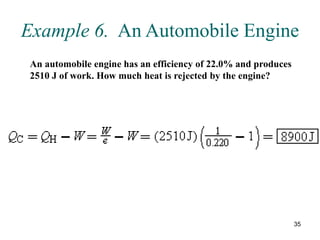
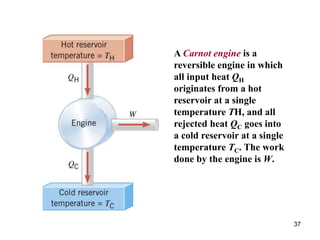
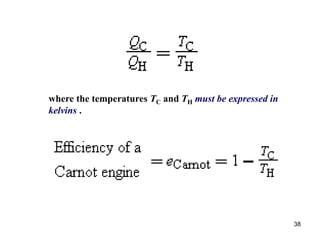
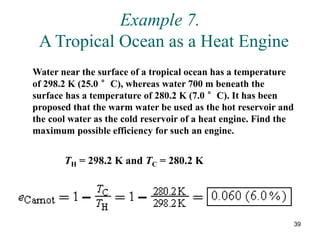
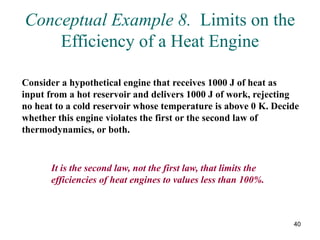
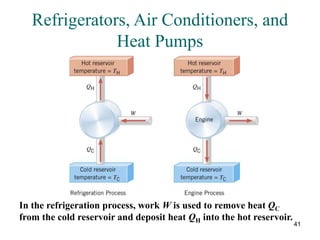
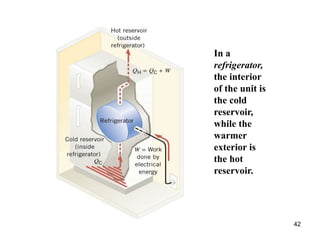
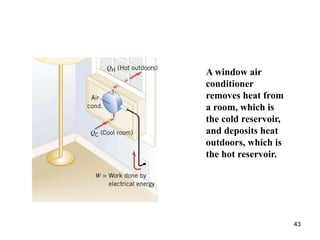
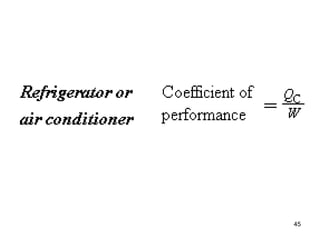
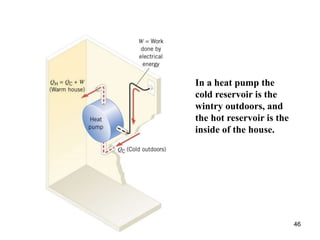
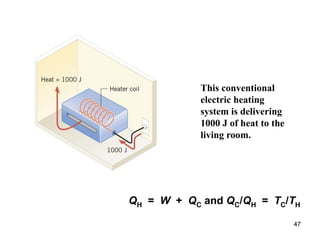
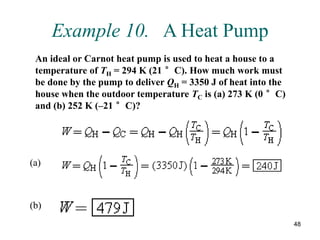
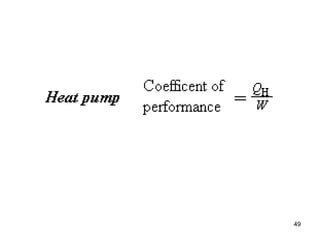
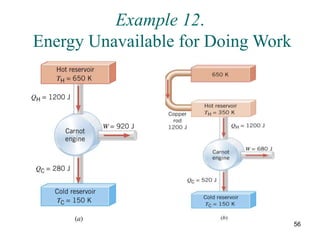
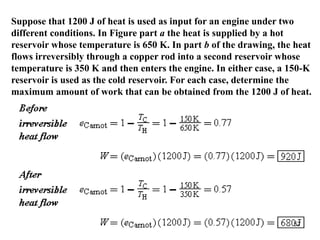
3) Heat engines use heat to perform work. The efficiency of heat engines is limited by the temperatures of the hot and cold reservoirs according to the Carnot efficiency formula. Refrigerators and heat pumps operate according to similar principles but use work to transfer
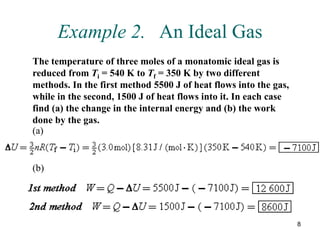
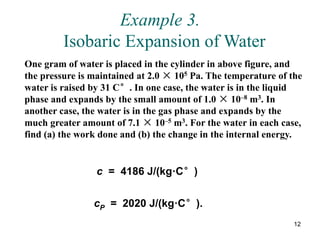

![14
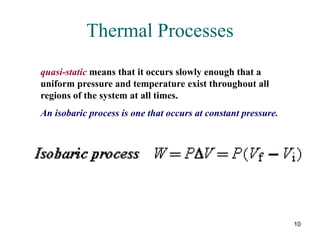
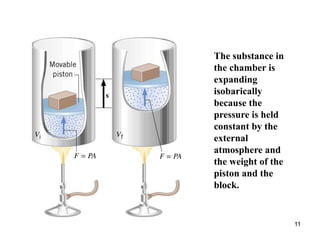
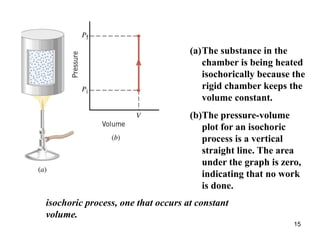
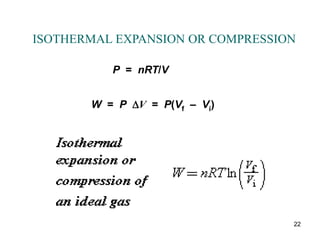
For an isobaric process, a pressure-versus-volume plot is a
horizontal straight line, and the work done [W = P(V f – V i)]
is the colored rectangular area under the graph.](https://image.slidesharecdn.com/ch15-231001131741-f5a680fa/85/ch15-ppt-14-320.jpg)

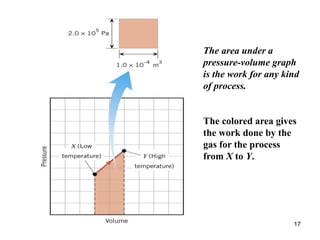
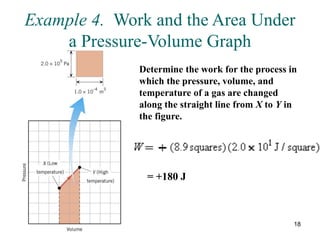
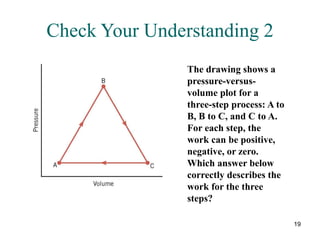
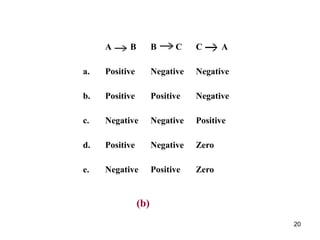
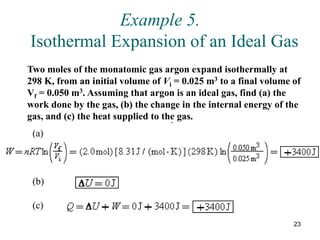
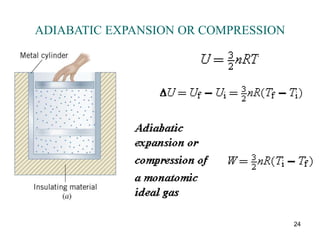
![25
[Ti = PiVi/(nR)]
[Tf = PfVf/(nR)].](https://image.slidesharecdn.com/ch15-231001131741-f5a680fa/85/ch15-ppt-25-320.jpg)
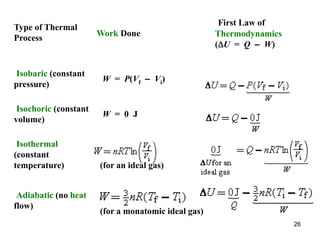
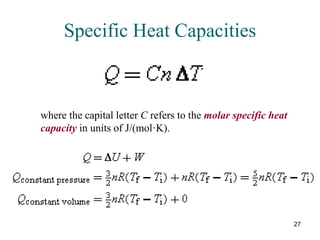





![70
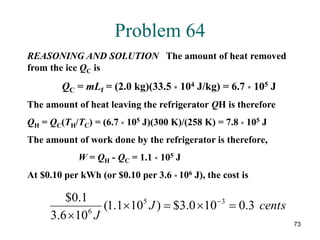
Problem 51
REASONING AND SOLUTION The efficiency of the engine
is e = 1 - (TC/TH) so
(i) Increase TH by 40 K; e = 1 - [(350 K)/(690 K)] = 0.493
(ii)Decrease TC by 40 K; e = 1 - [(310 K)/(650 K)] = 0.523
The greatest improvement is made by lowing the temperature
of the cold reservoir.](https://image.slidesharecdn.com/ch15-231001131741-f5a680fa/85/ch15-ppt-70-320.jpg)

V
Q Q
T
C n R n
](https://image.slidesharecdn.com/ch15-231001131741-f5a680fa/85/ch15-ppt-72-320.jpg)