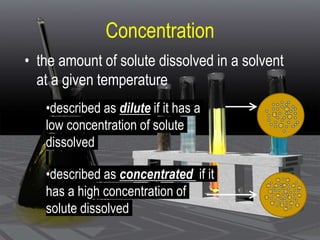
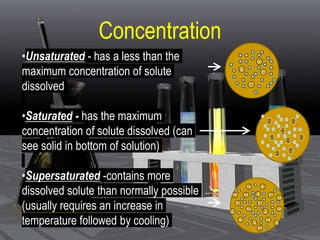
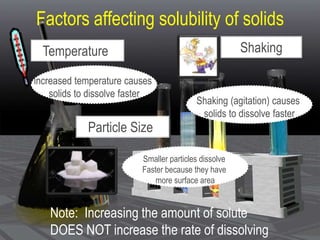
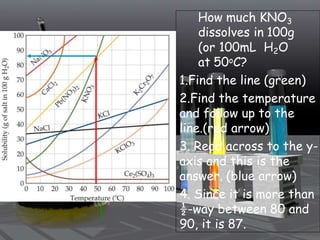
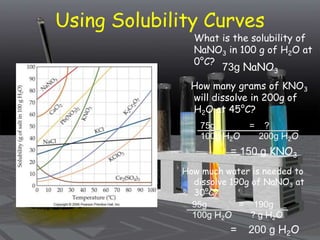
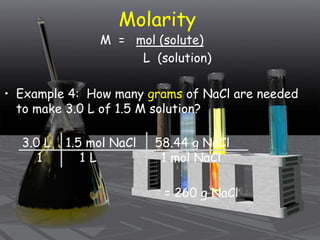
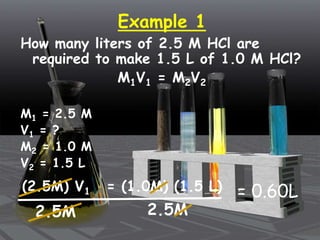
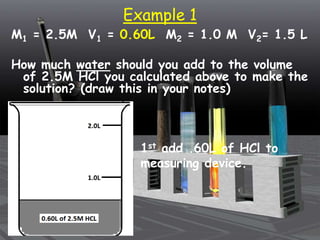
This document provides an overview of solutions and related concepts. It defines heterogeneous and homogeneous mixtures, noting that solutions are homogeneous mixtures composed of solutes and solvents. Key terms discussed include concentration, saturation, solubility factors, and polarity. Solution types include liquid, solid, and gas solutions. Concentration can be expressed using molarity, percent by mass, or molality. Dilutions and solution stoichiometry problems are also reviewed.