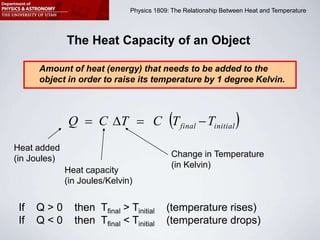
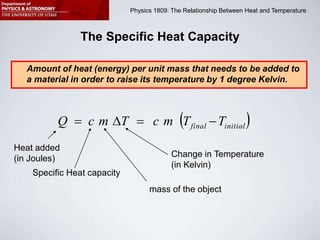
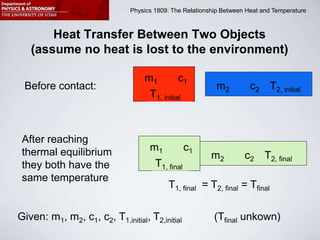
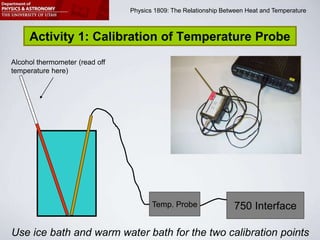
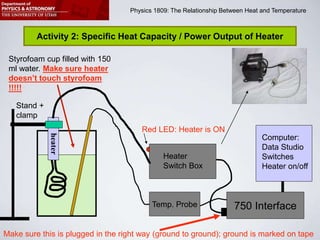
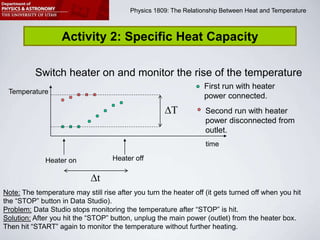
This document describes four activities to measure heat transfer and specific heat capacities:
1) Calibrate a temperature probe using ice and warm water baths.
2) Determine the power output of a heating element by measuring temperature changes in heated water.
3) Measure the specific heat of isopropyl alcohol using the calibrated heater power.
4) Determine the specific heat of brass by cooling a disc in liquid nitrogen and monitoring its temperature increase in water.