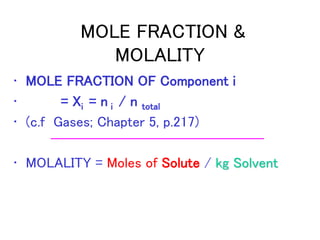
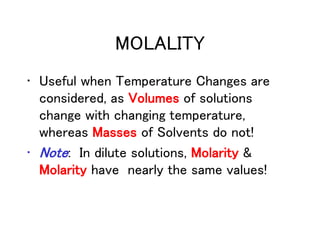
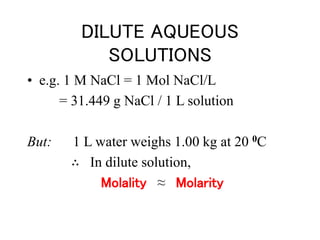
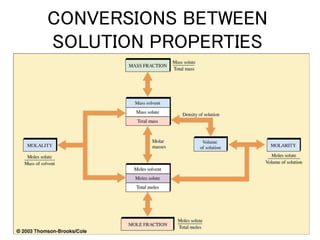
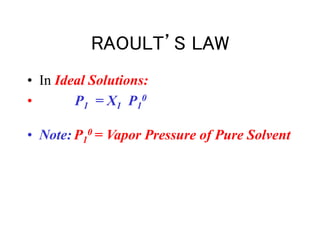
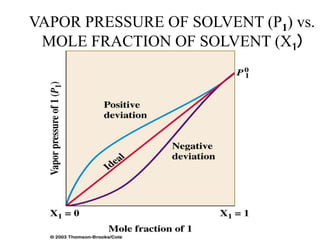
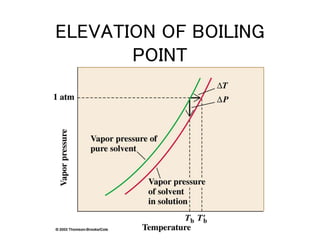
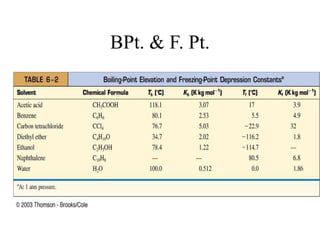
This document discusses several colligative properties of solutions including boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. It defines key terms like mole fraction, molality, and van't Hoff factor. It explains that in dilute solutions, molality and molarity are nearly equivalent. The document also covers conversions between solution properties, Raoult's law, and how dissociation of solutes is related to the van't Hoff factor and impacts freezing point depression and boiling point elevation.


![Van’t HOFF FACTOR
Δ Tf = imK f
i = No. of particles in solution per formula
unit (range 1 – n)
i.e. for sucrose i = 1 [no dissociation]
for NaCl i = 2 [NaCl → Na++Cl-]
for K2SO4 i = 3 [K2SO4 → 2K+ + SO4
2- ]](https://image.slidesharecdn.com/chapter-6-colligative-properties-230814034832-b8db48f0/85/Chapter-6-Colligative-Properties-ppt-11-320.jpg)

