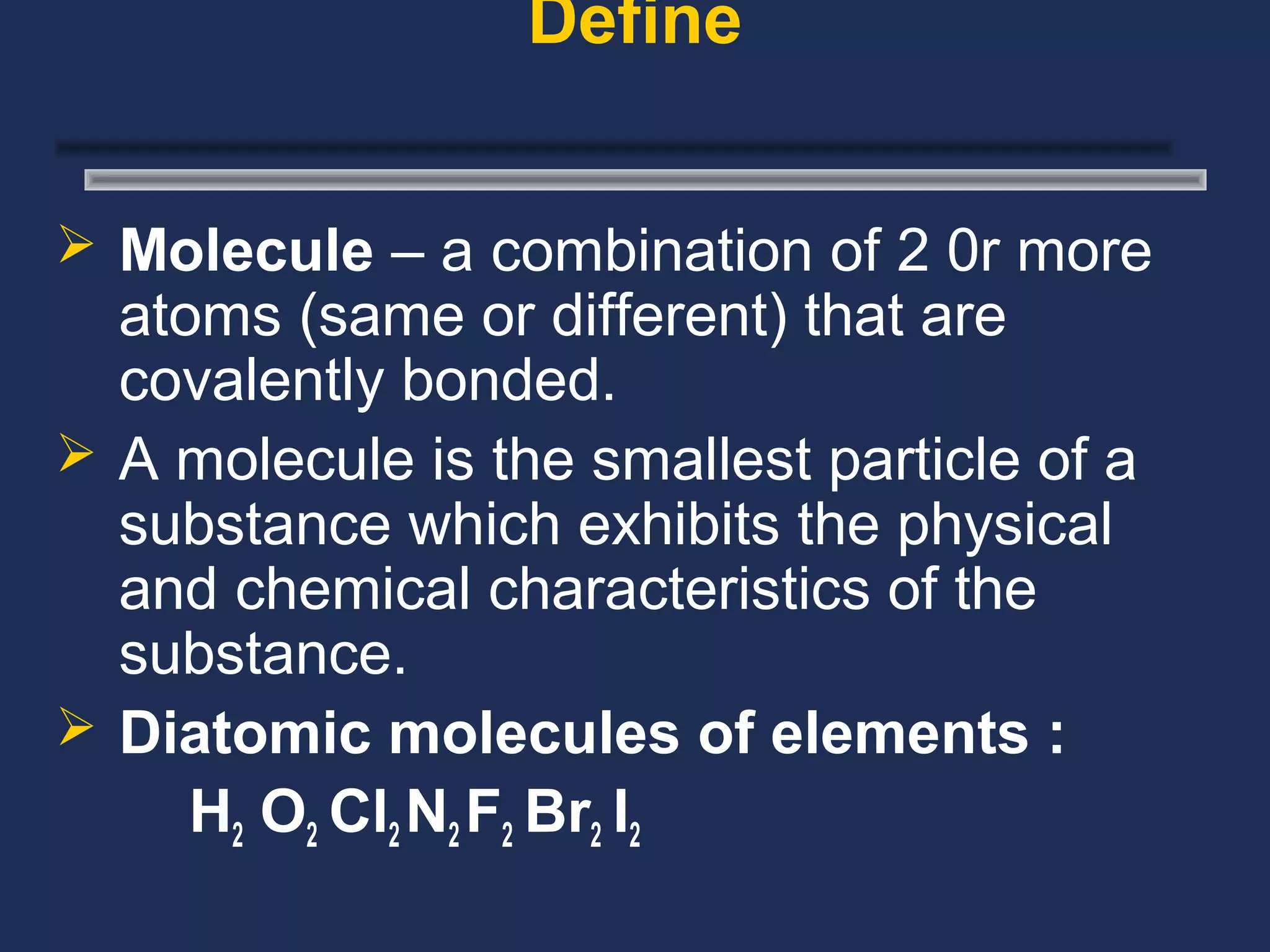
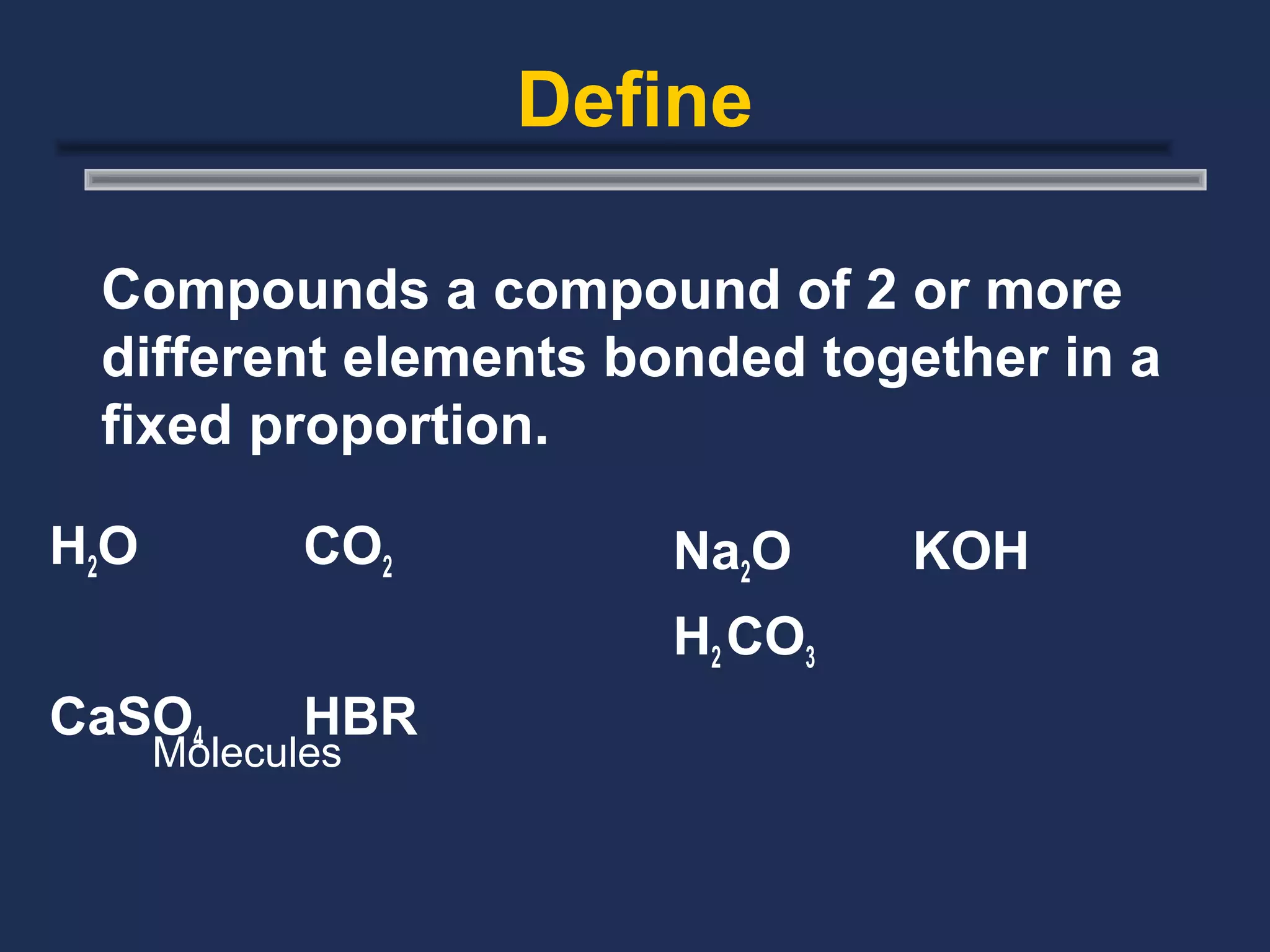
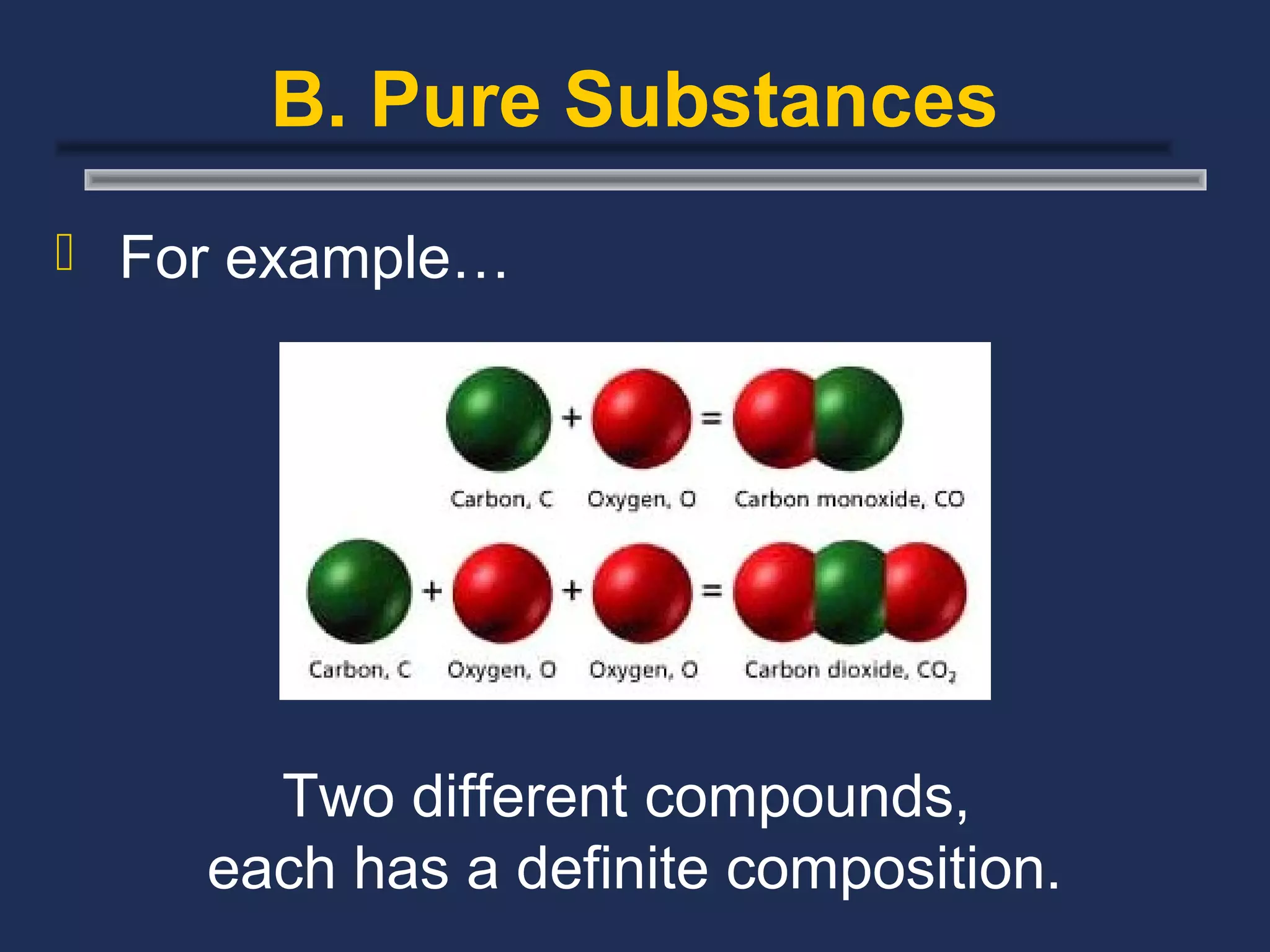
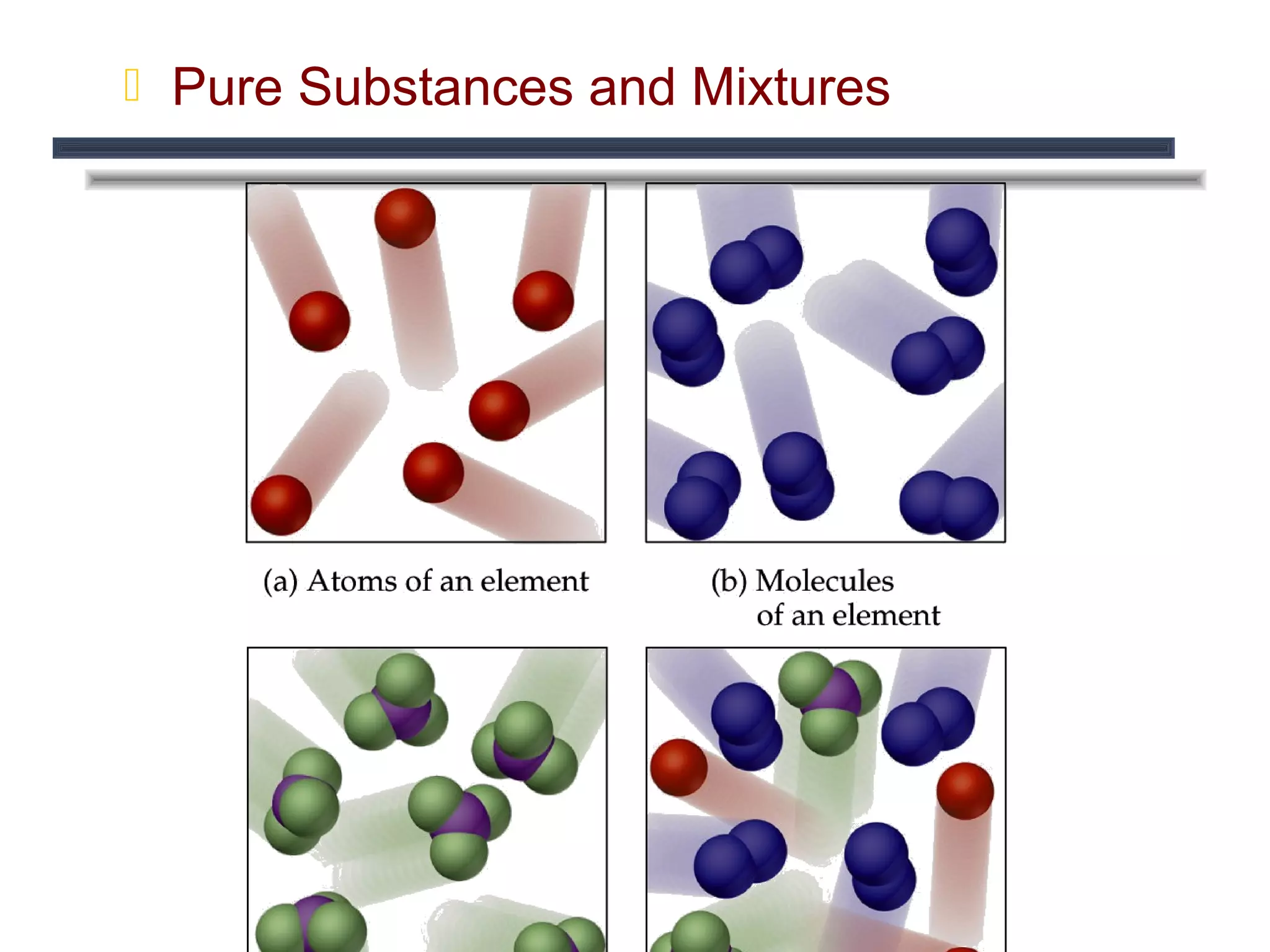
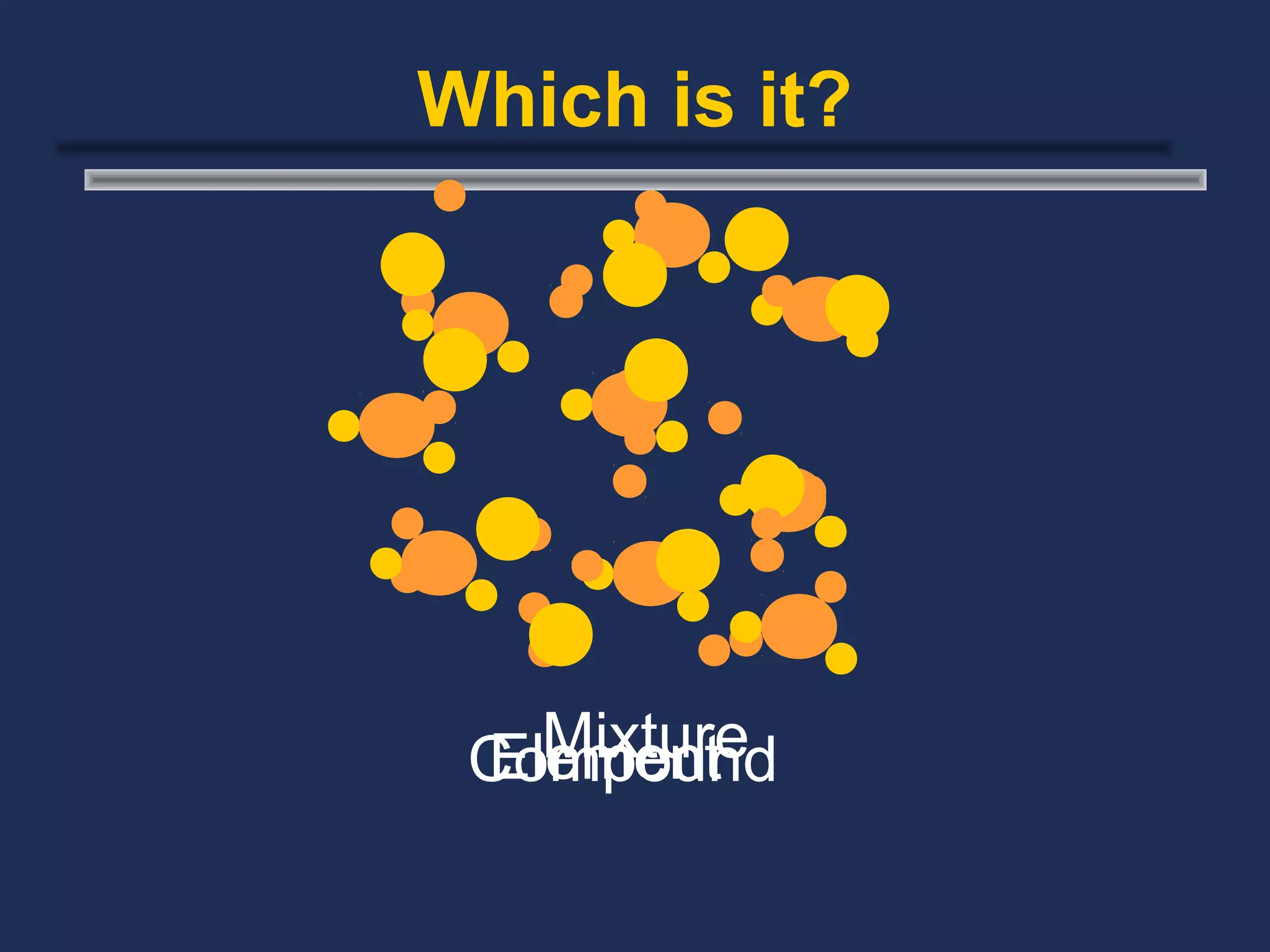
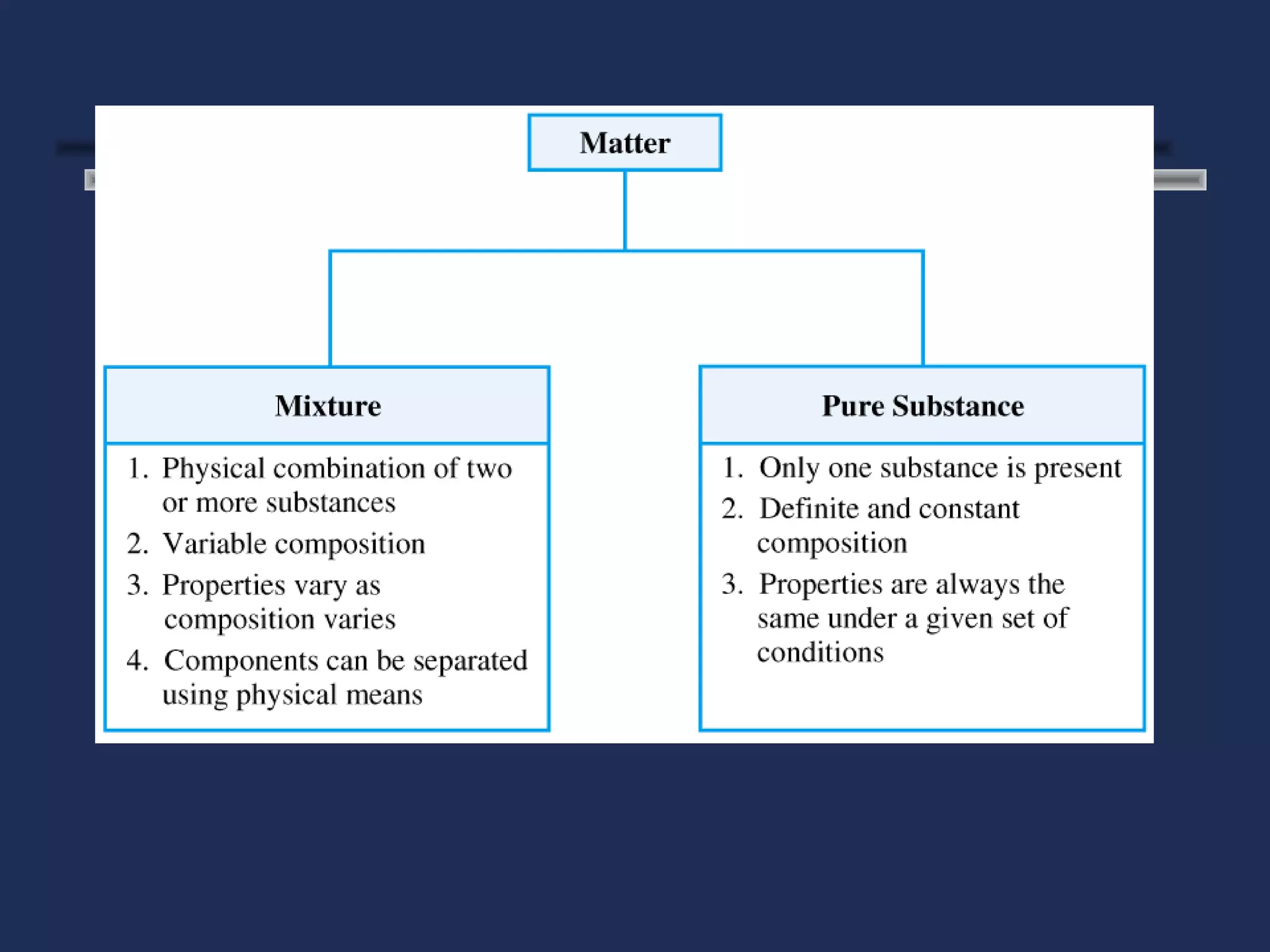
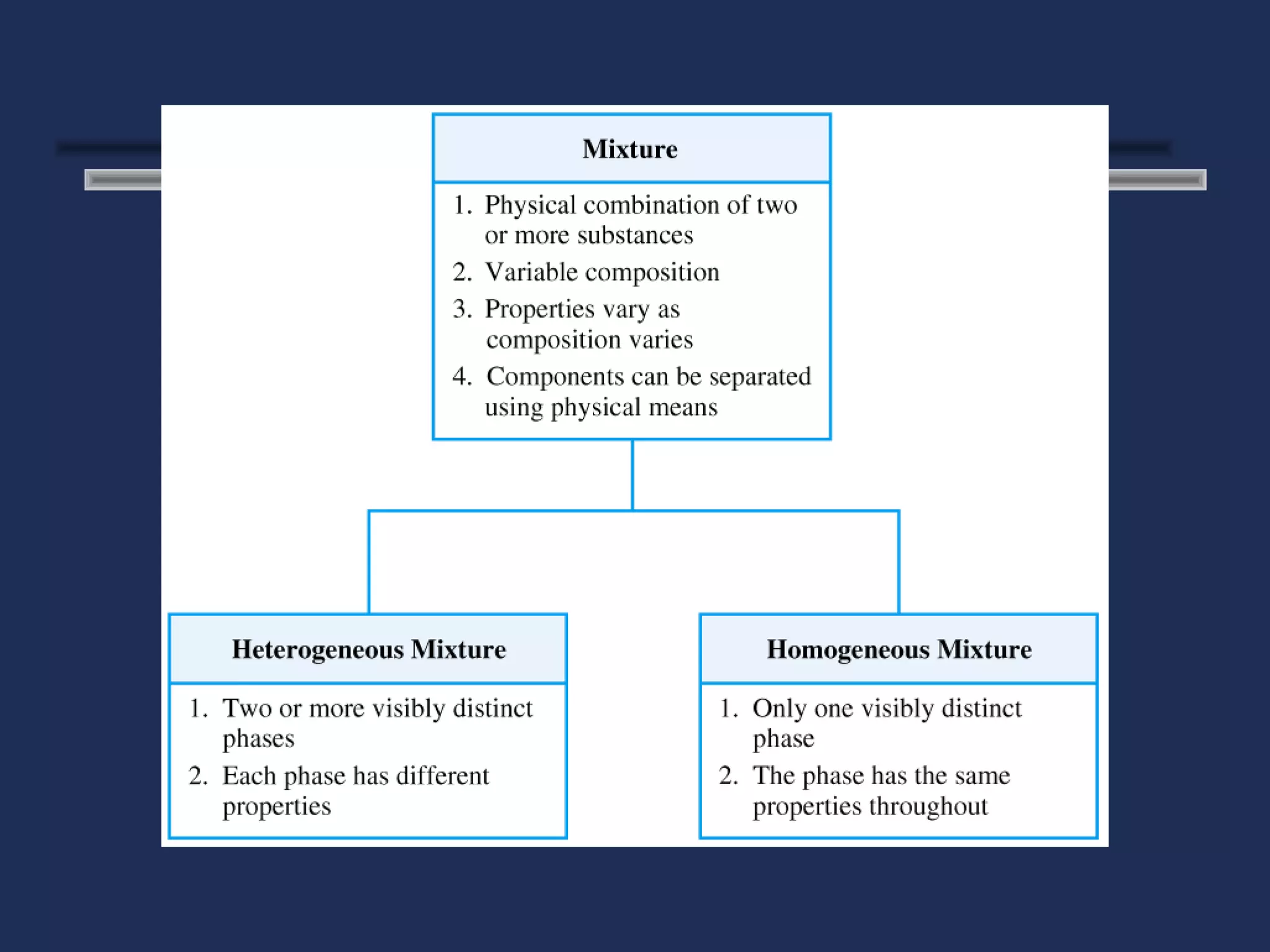
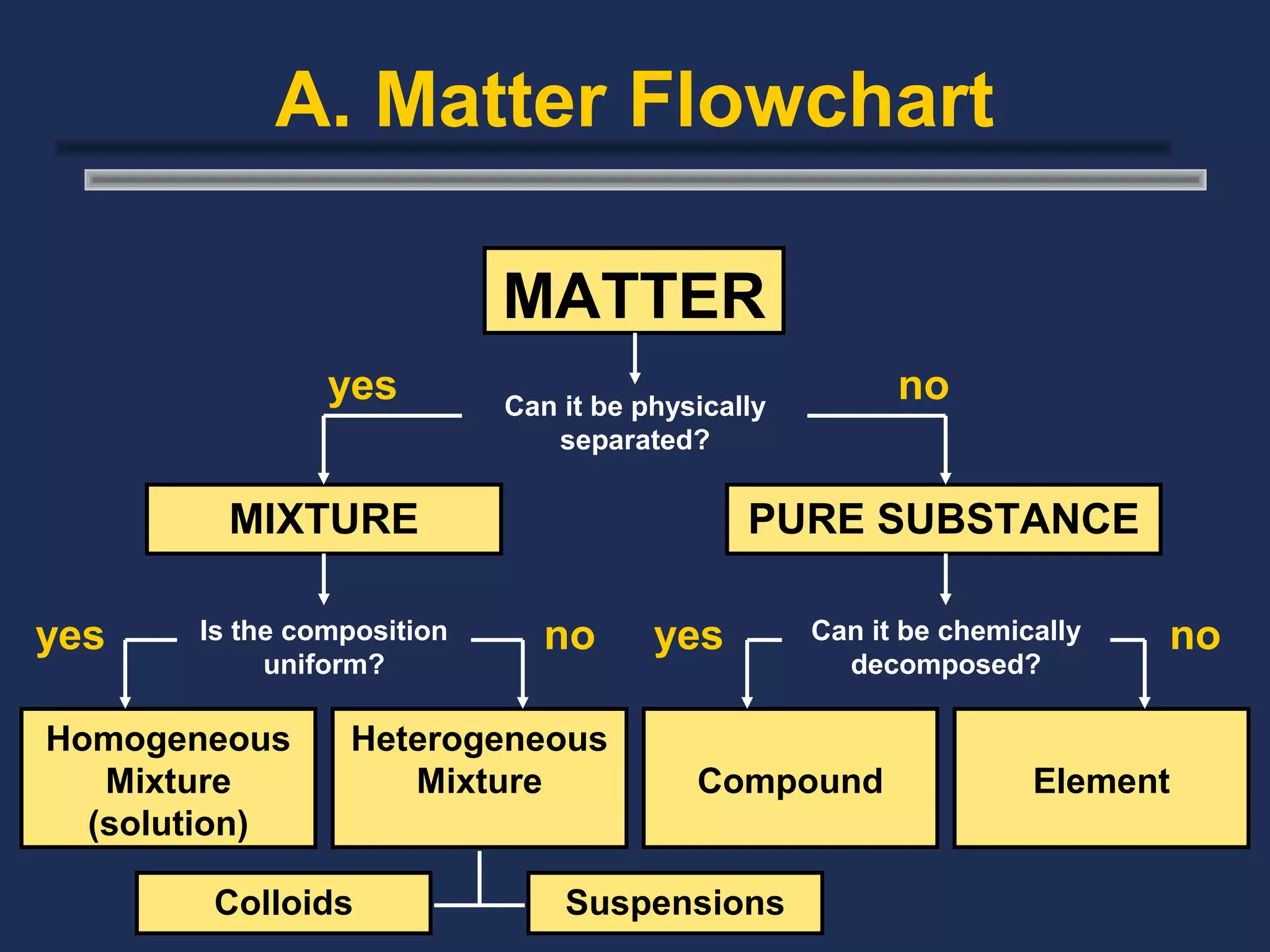
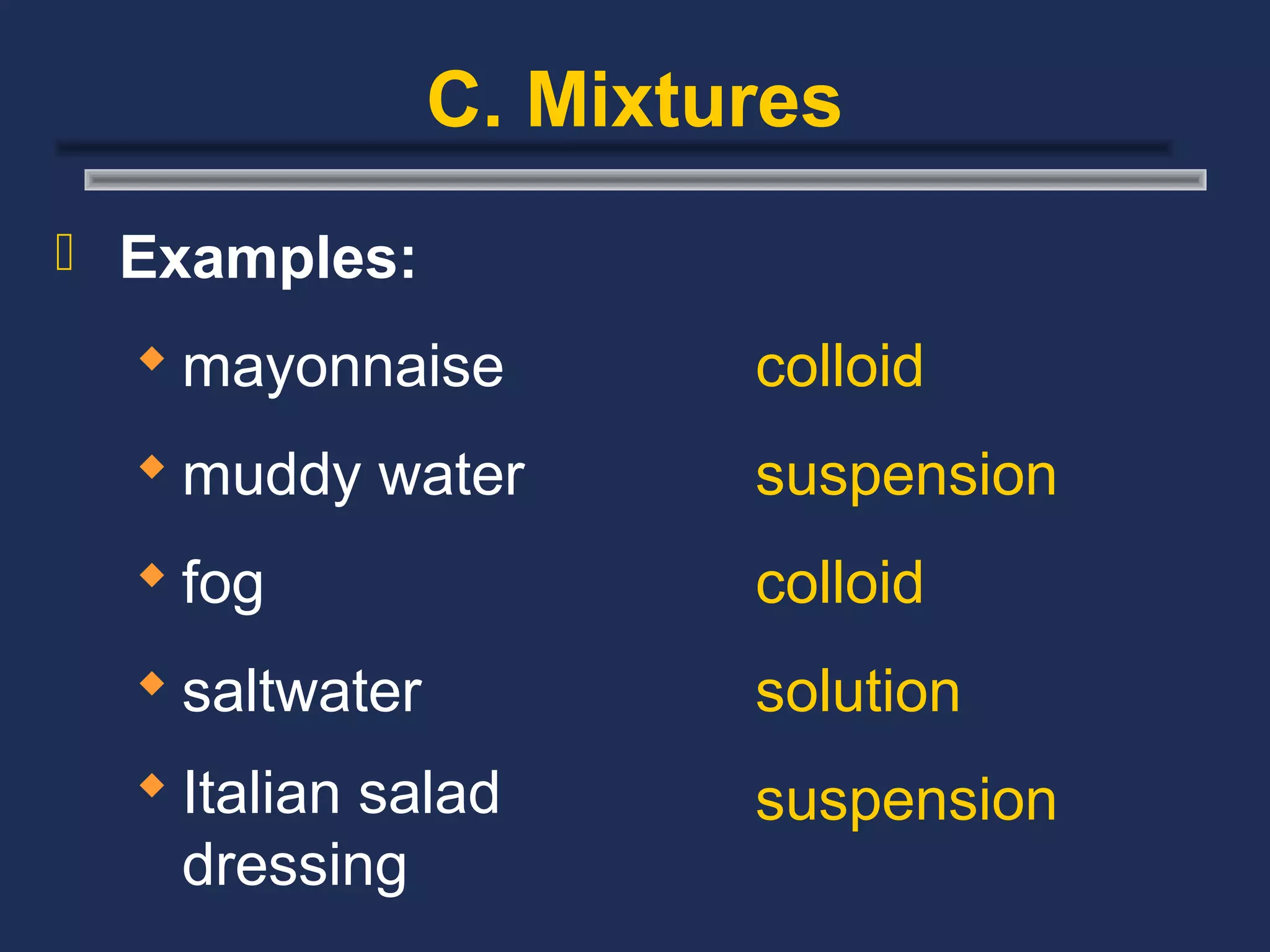
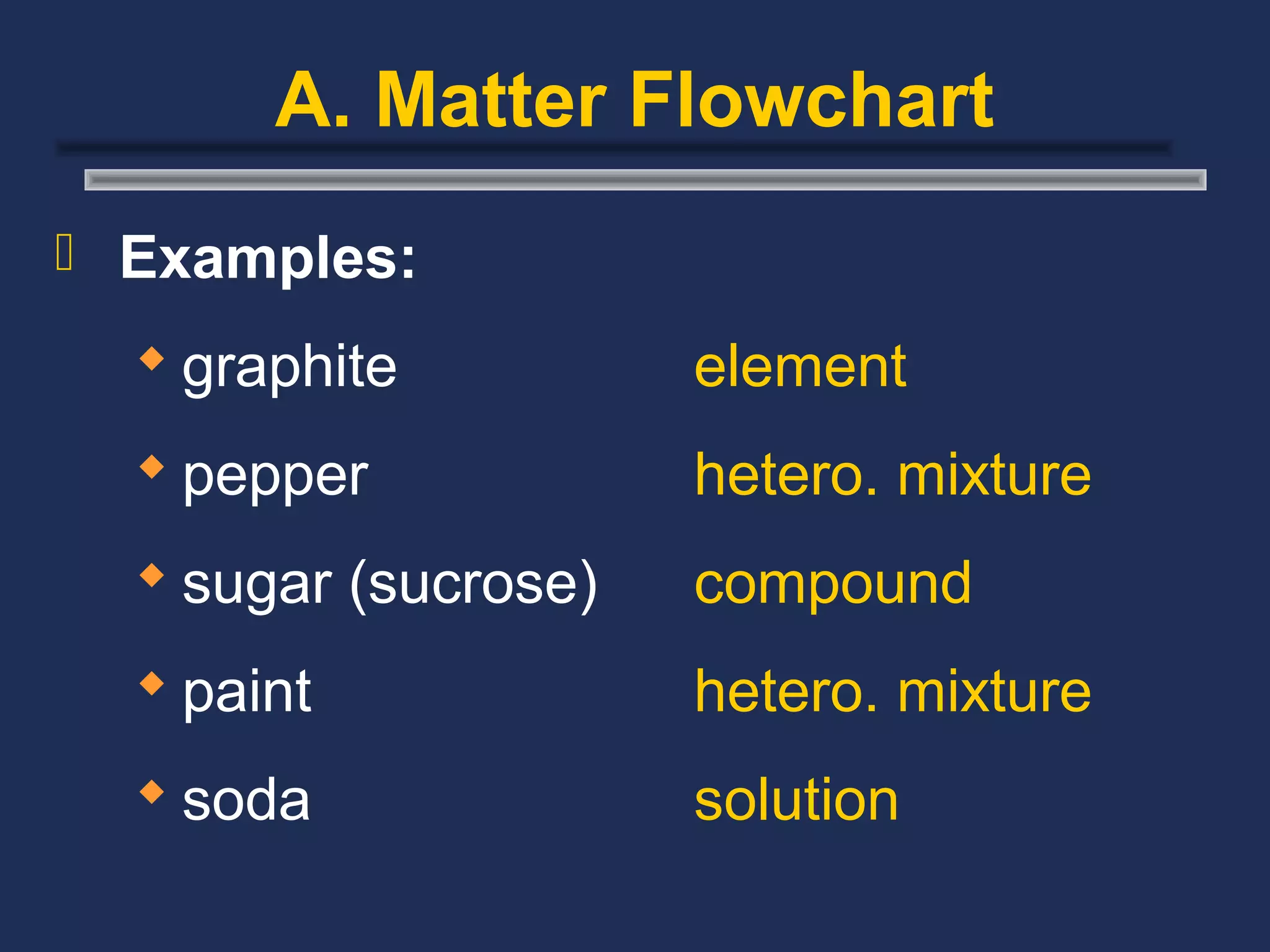
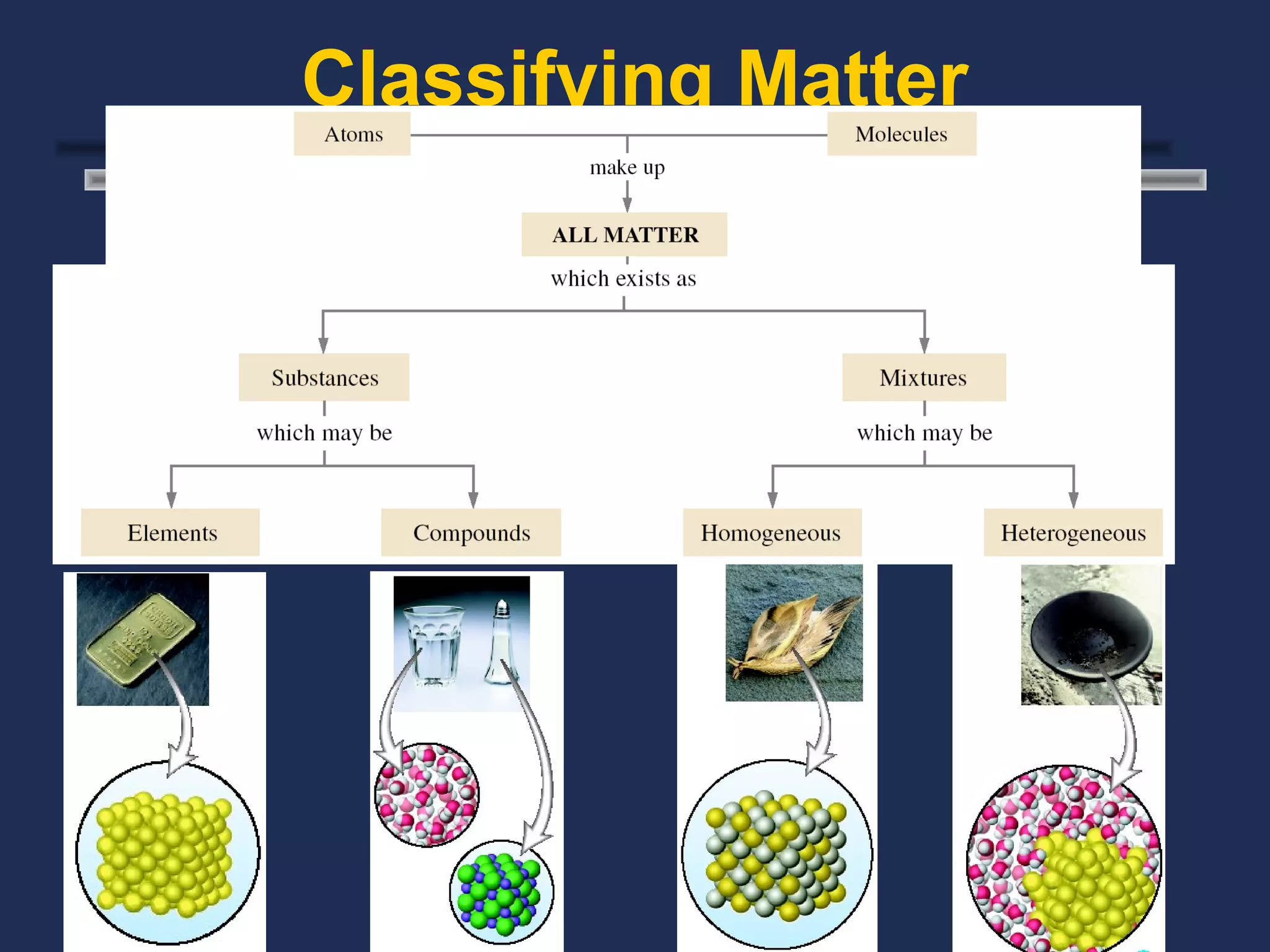
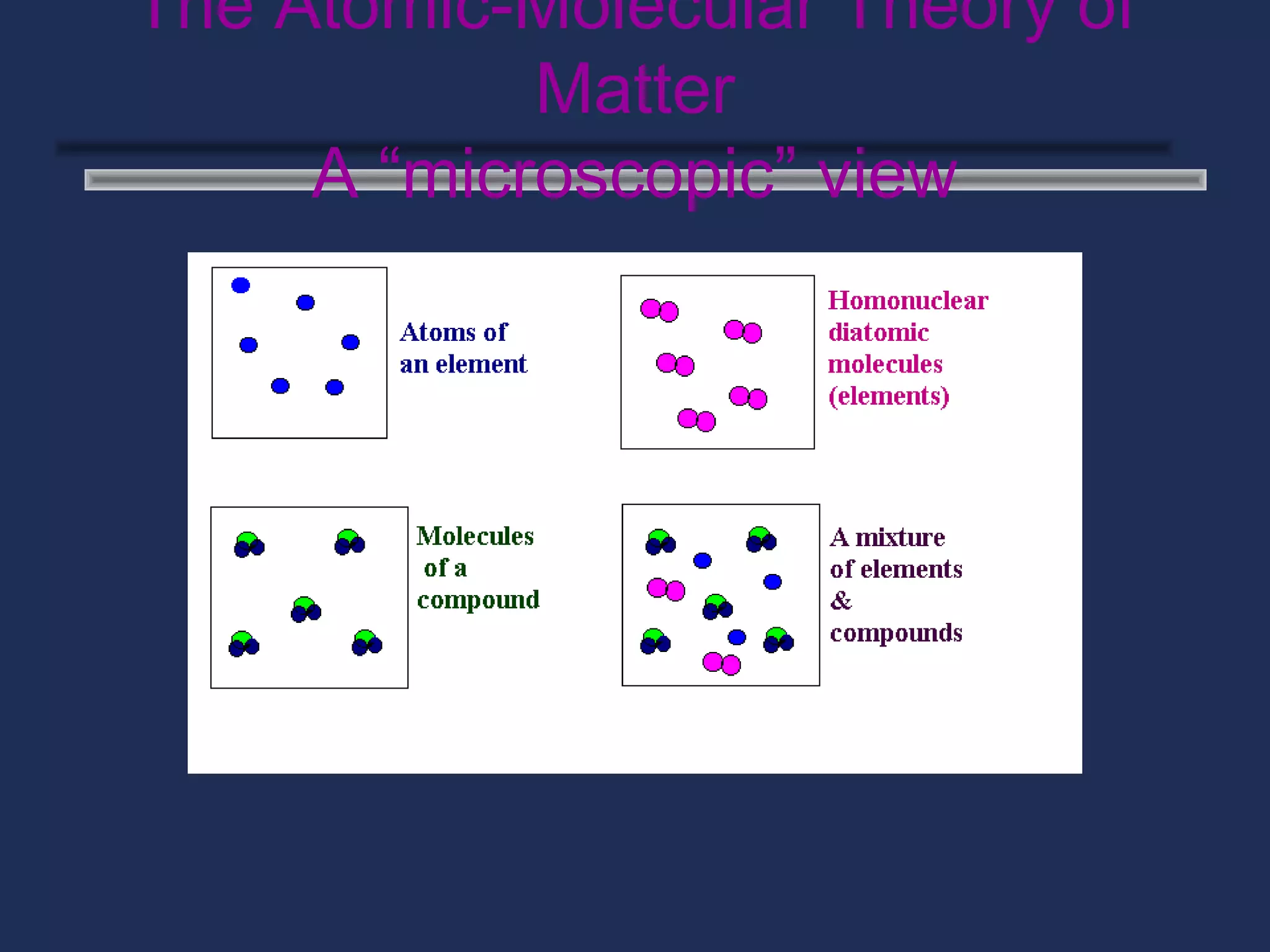
The document outlines the classification of matter, defining key concepts such as atoms, elements, compounds, and mixtures. It distinguishes between pure substances and mixtures, explaining their properties and how they can be separated. Various types of mixtures and their characteristics, along with examples, are also provided to illustrate the differences between homogeneous and heterogeneous mixtures.