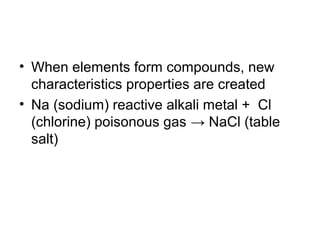
This document defines and provides examples of mixtures, solutions, suspensions, colloids, and gas mixtures. It explains that mixtures can be separated physically but their compositions are not fixed, while solutions appear homogeneous but are mixtures that dissolve. Suspensions and colloids are mixtures where particles settle or scatter light differently. The document also defines elements as pure substances that cannot be broken down, and compounds as pure substances formed by chemical combination of elements in specific ratios producing new properties.