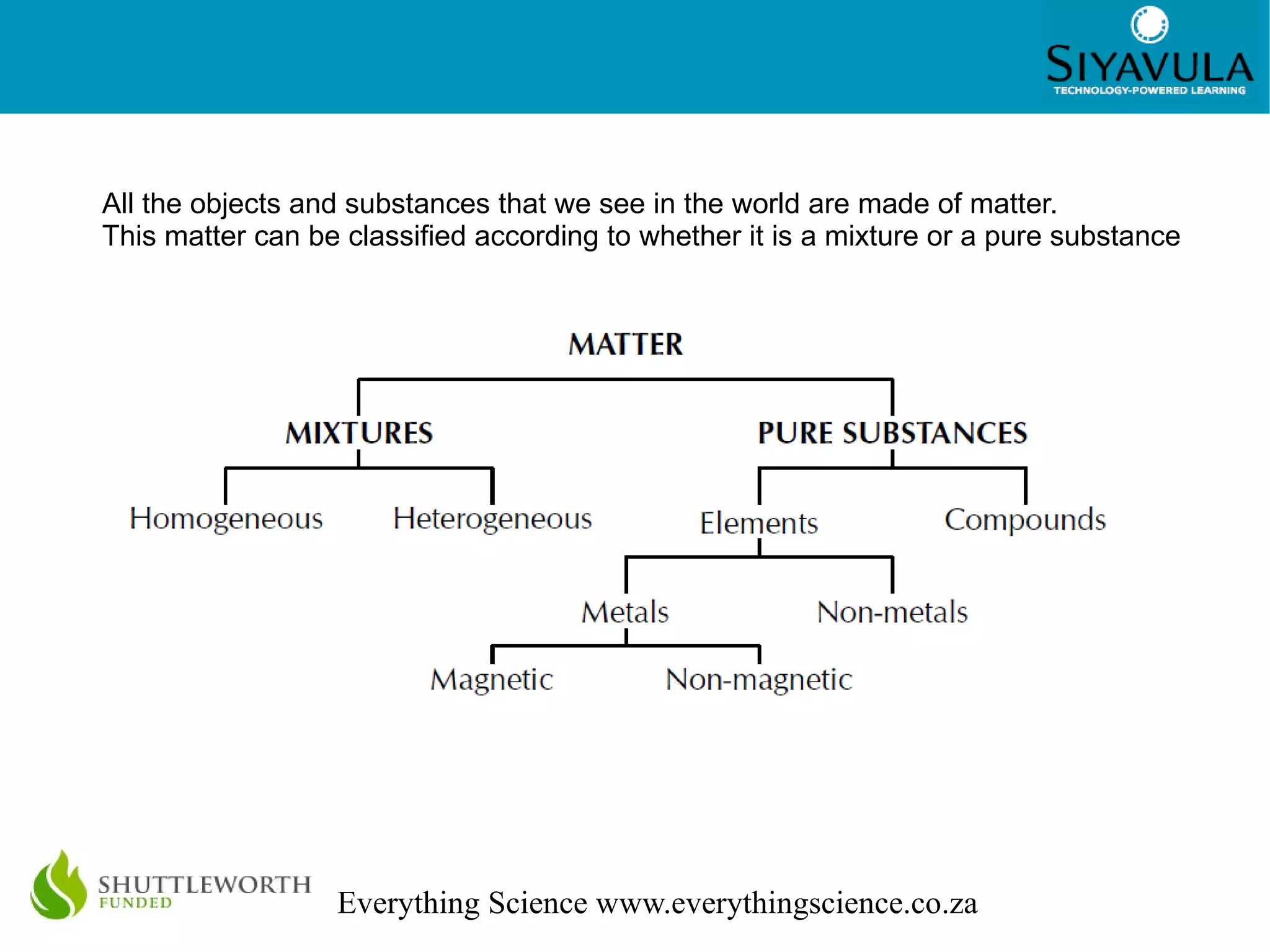
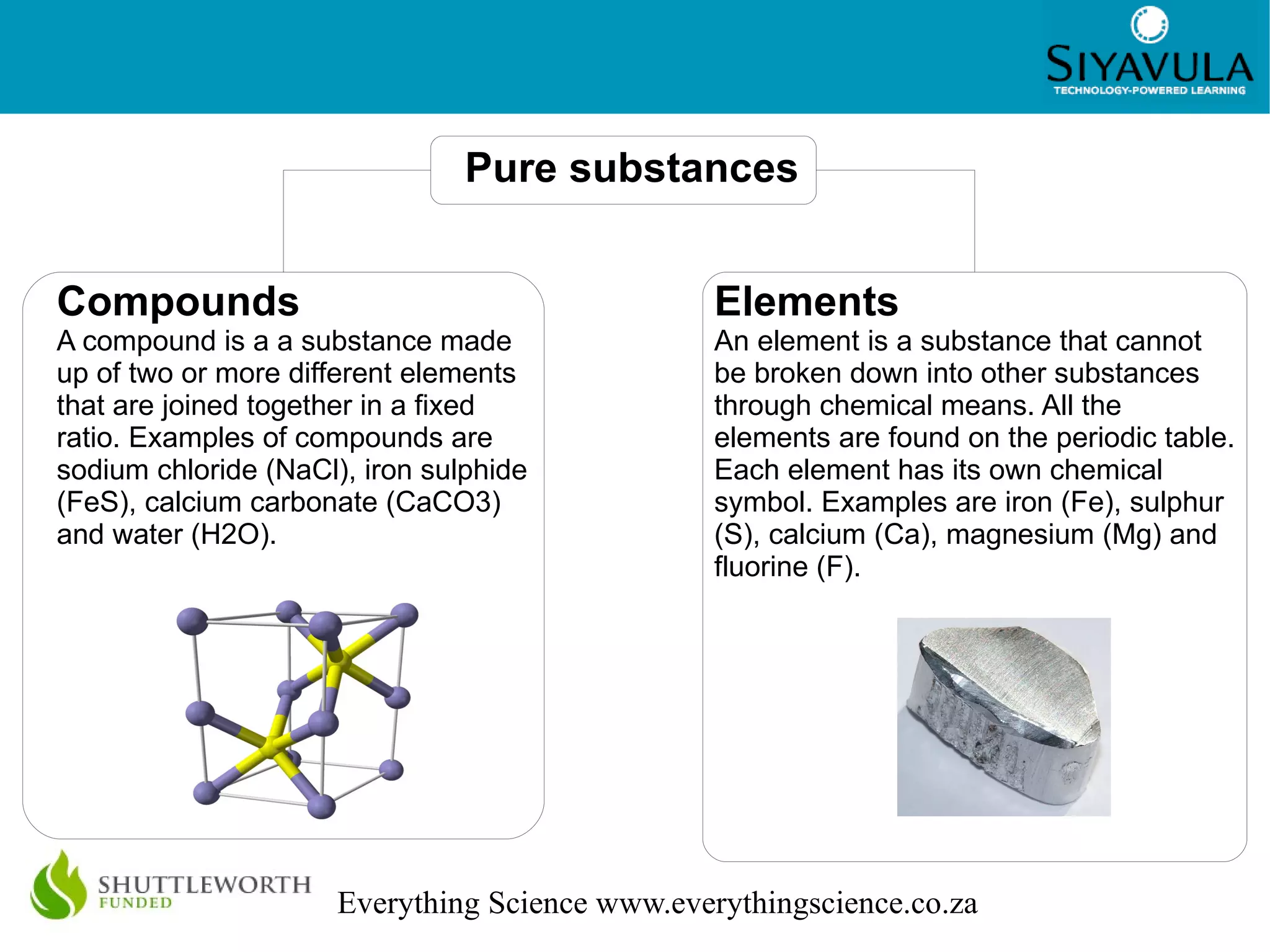
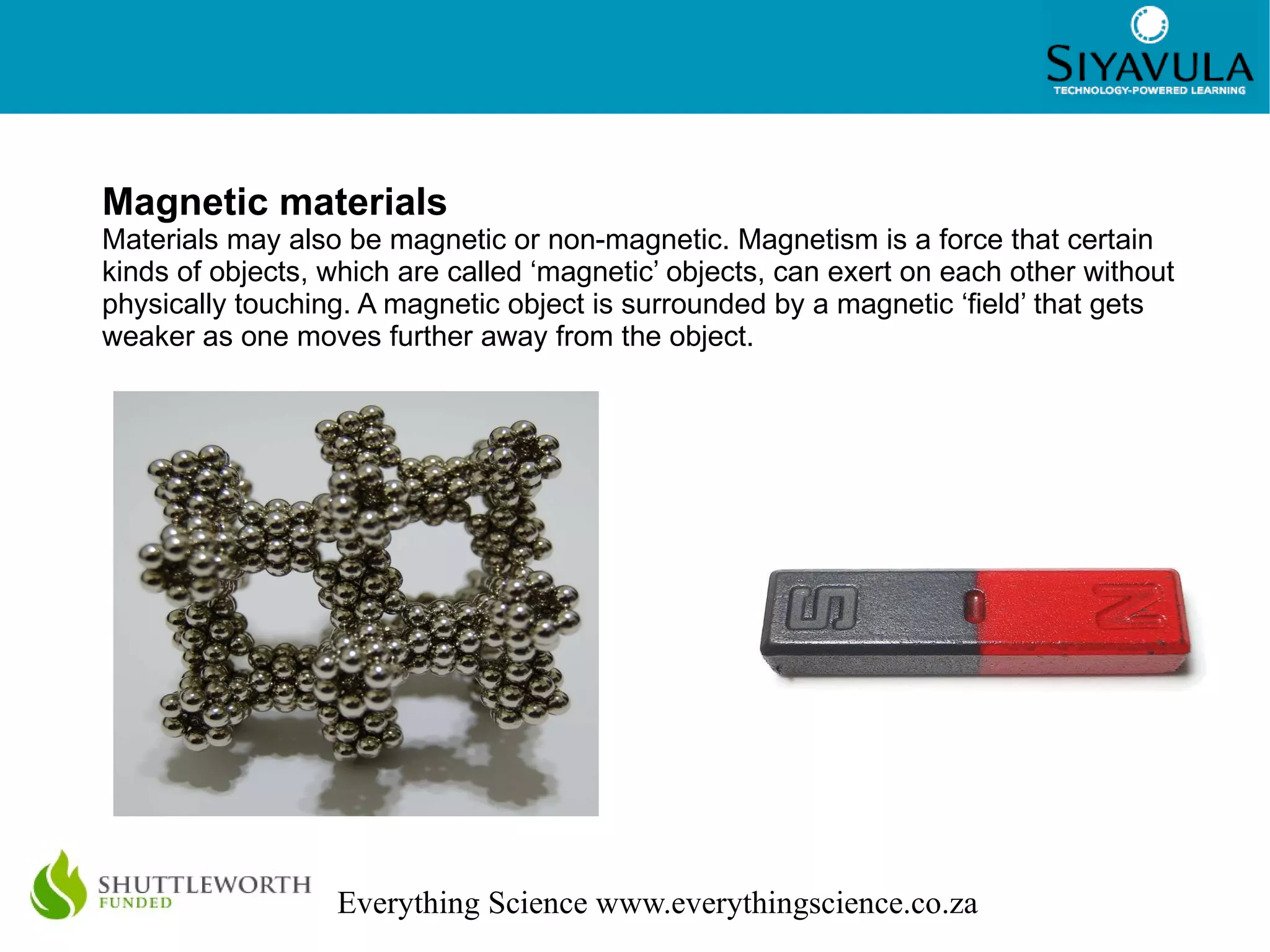
This document discusses the classification of matter. It defines mixtures as combinations of two or more substances that are not chemically bonded and can be separated by mechanical means. Mixtures can be heterogeneous, with visible components, or homogeneous, appearing uniform. Pure substances cannot be broken down and include elements, which cannot be broken down further, and compounds, made of two or more elements joined in a fixed ratio. Elements are found on the periodic table and compounds are named according to the elements present and their ratios. The properties of materials, such as their conductivity, magnetism, and position on the periodic table, determine whether they are classified as metals, nonmetals, or metalloids.