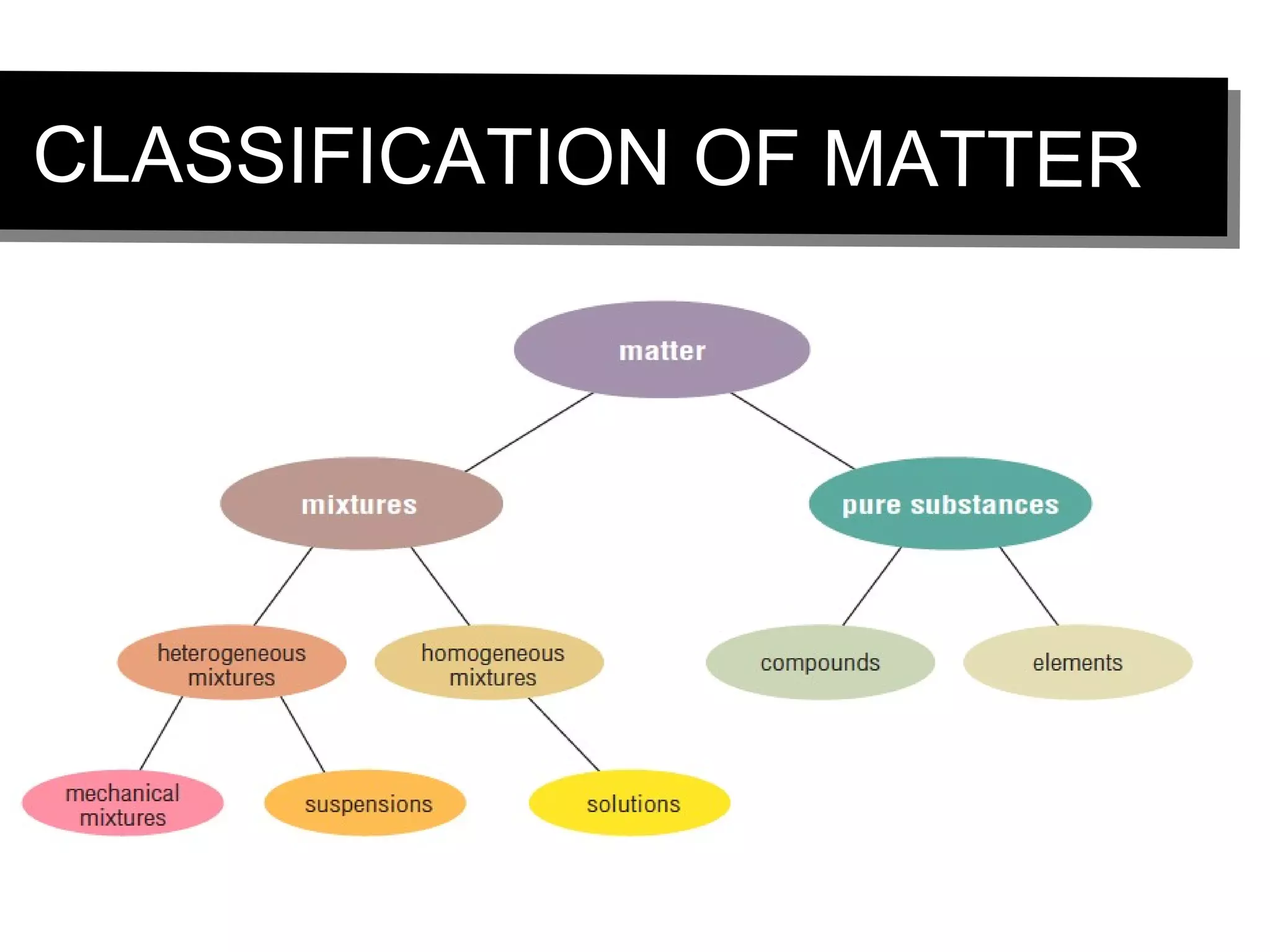
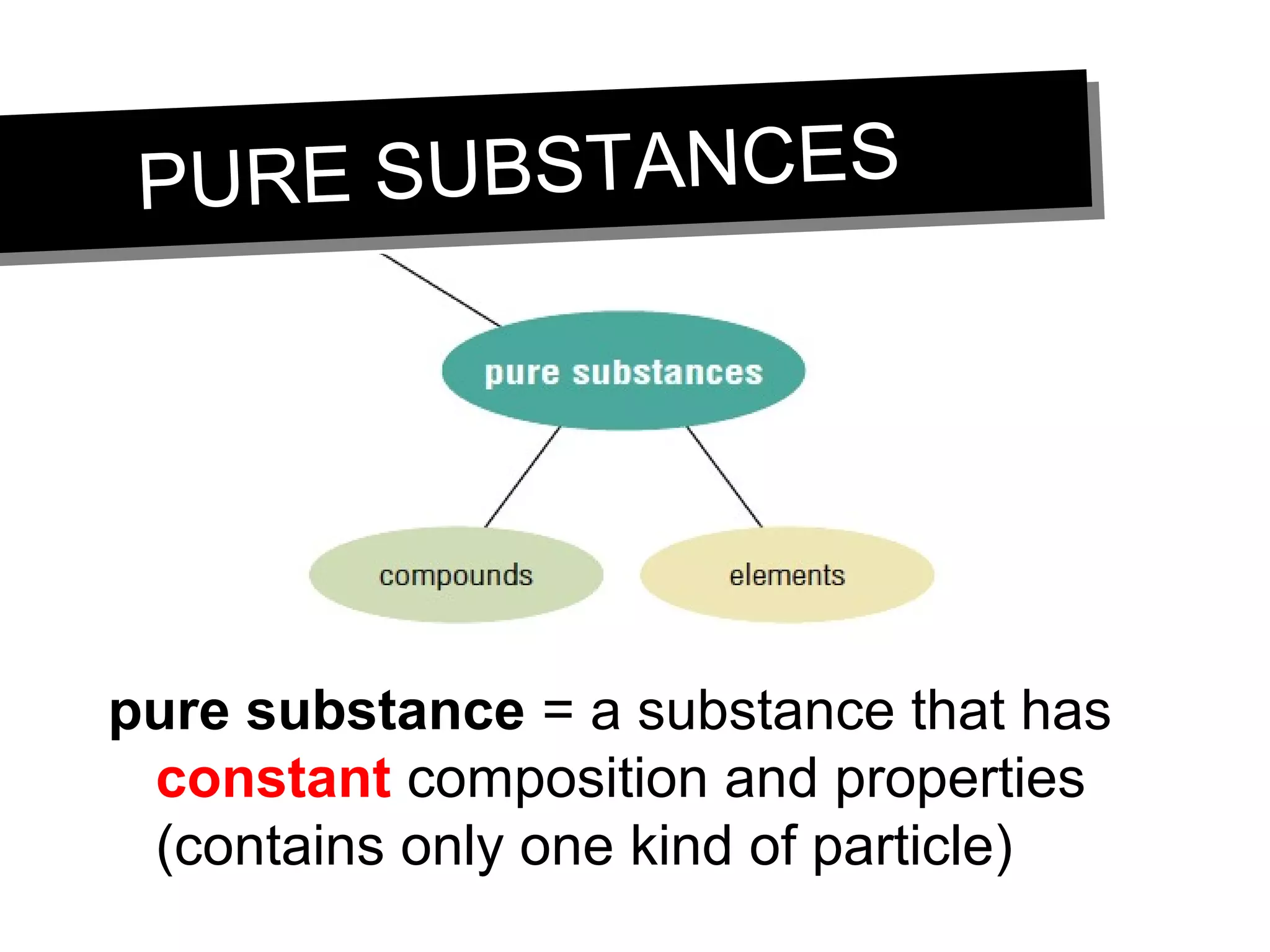
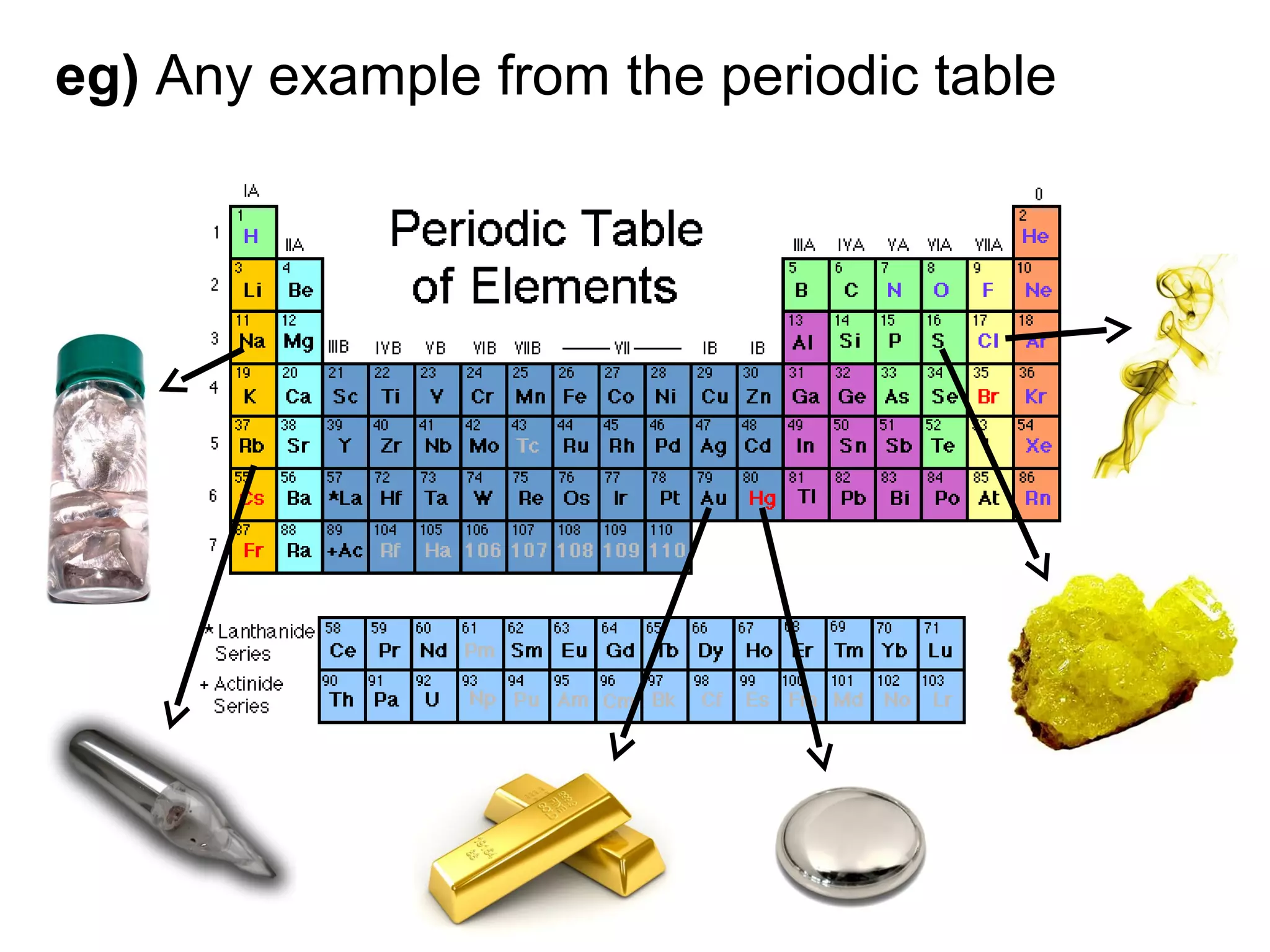
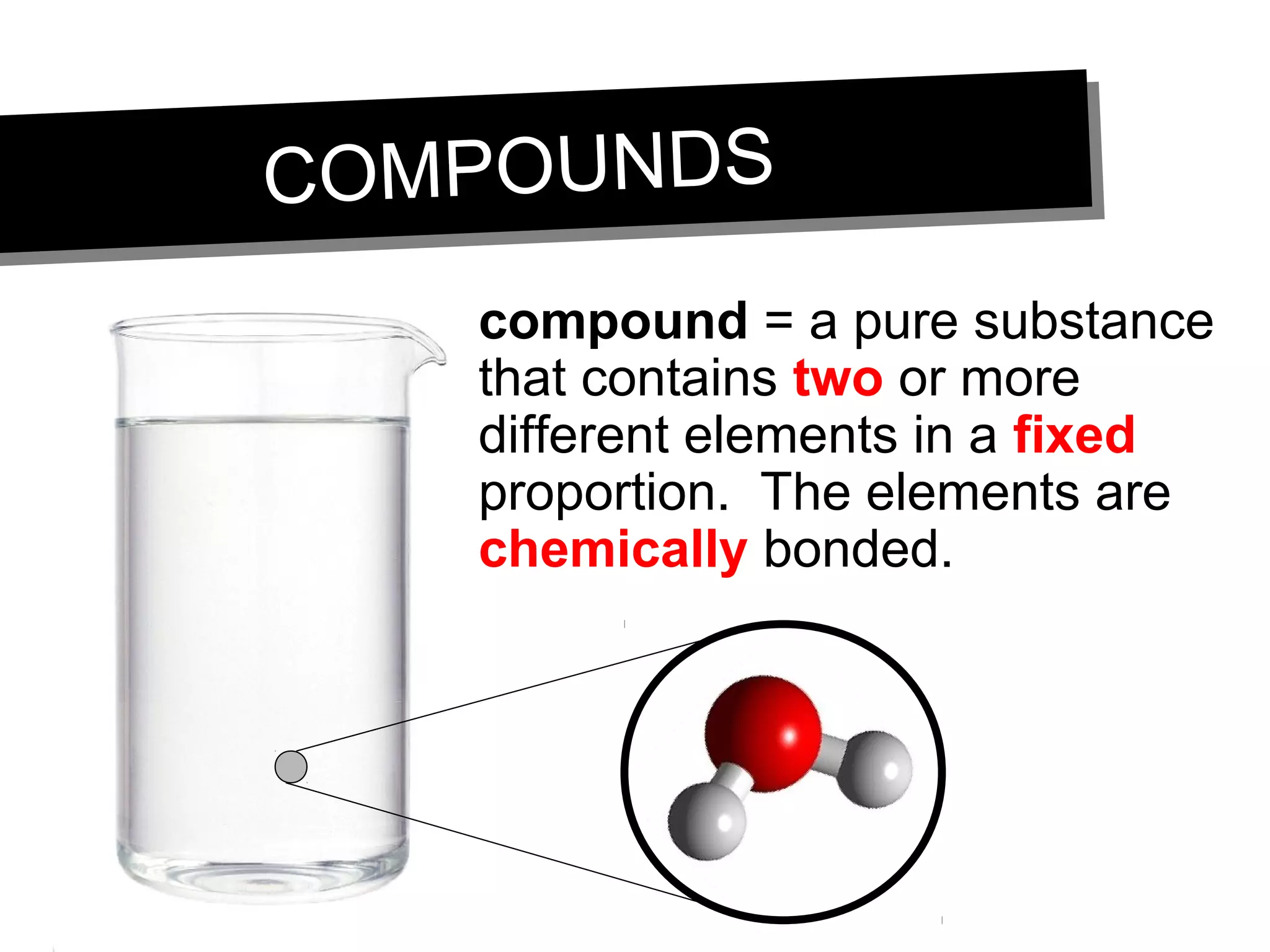
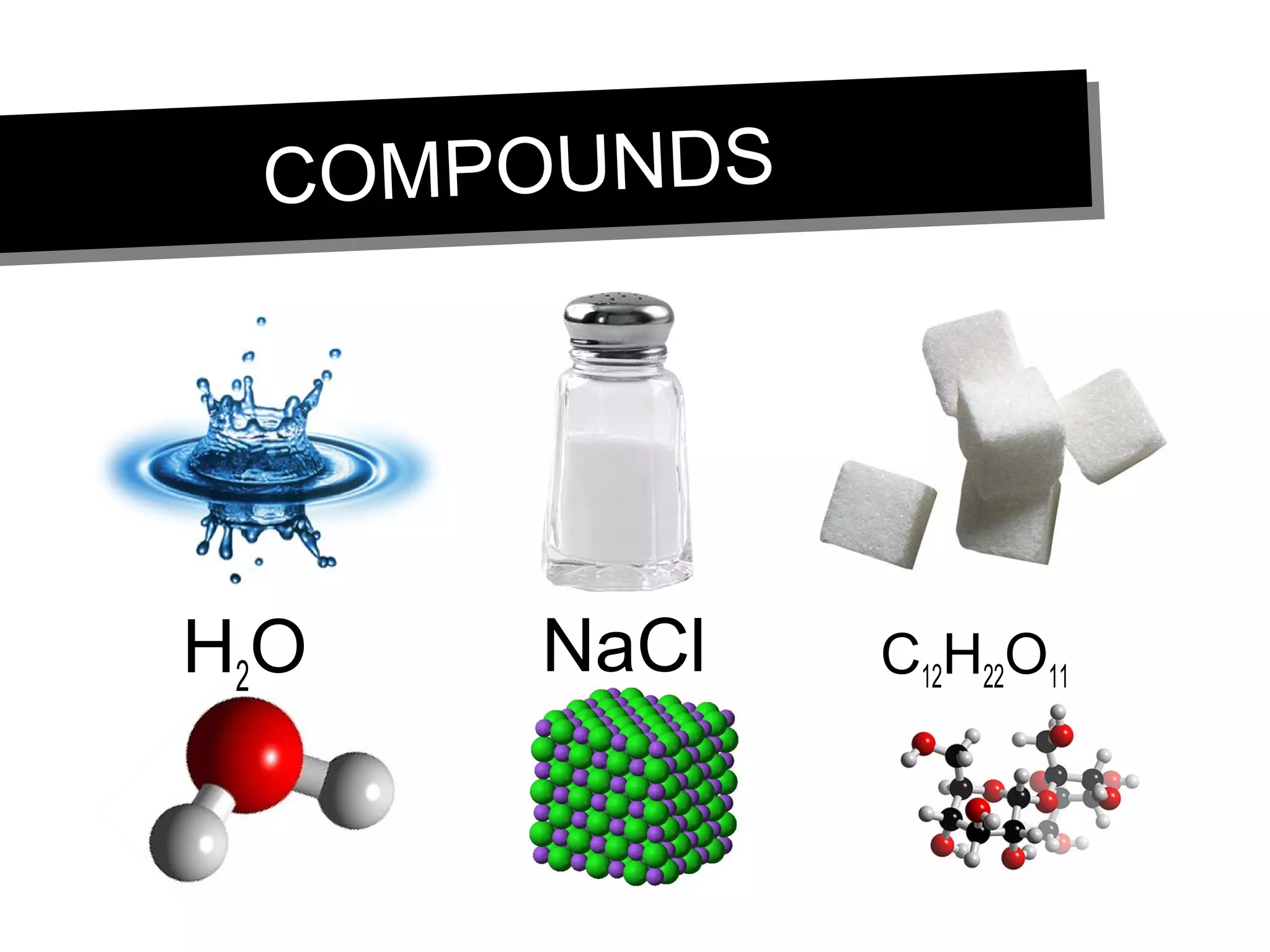
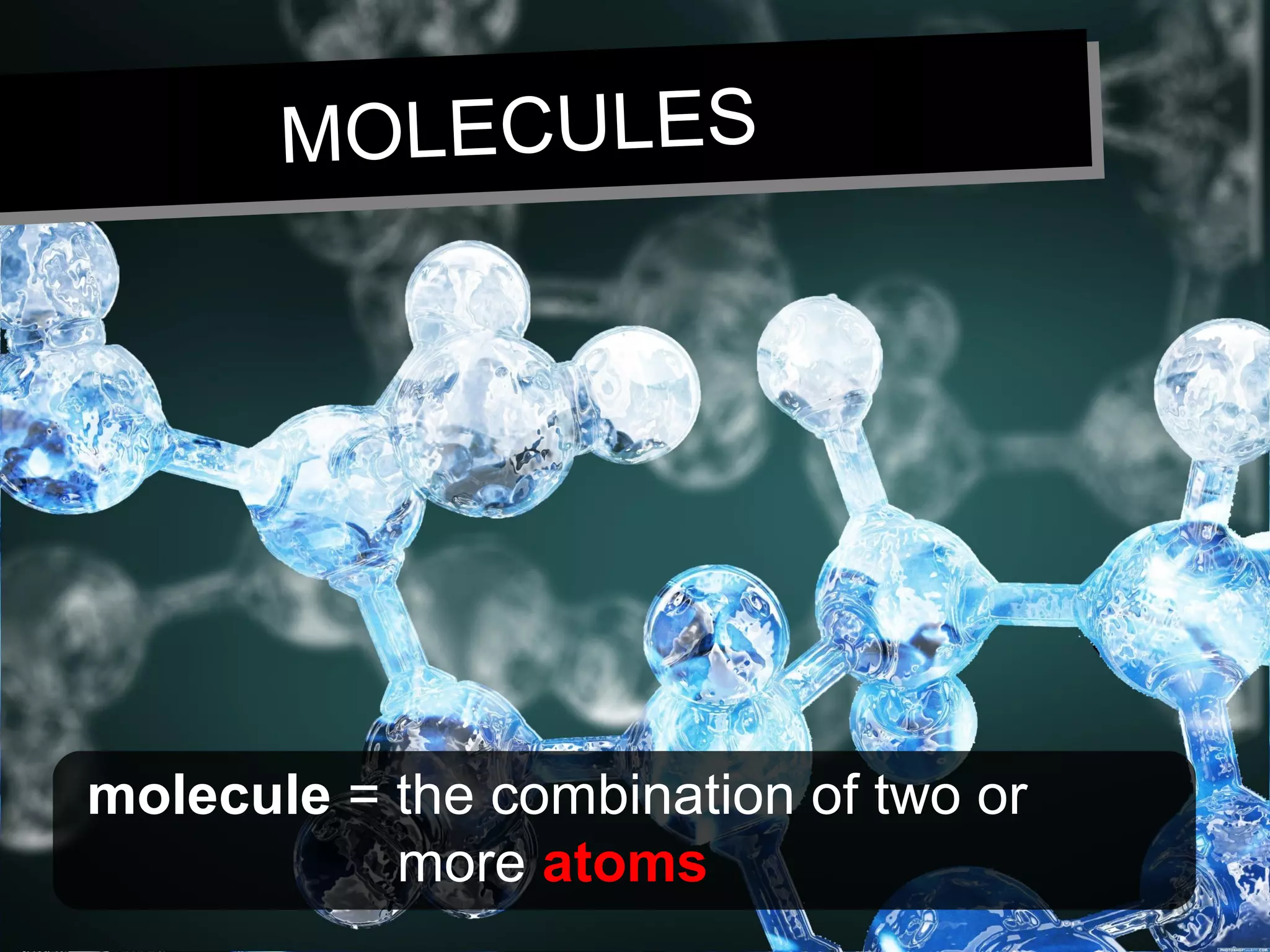
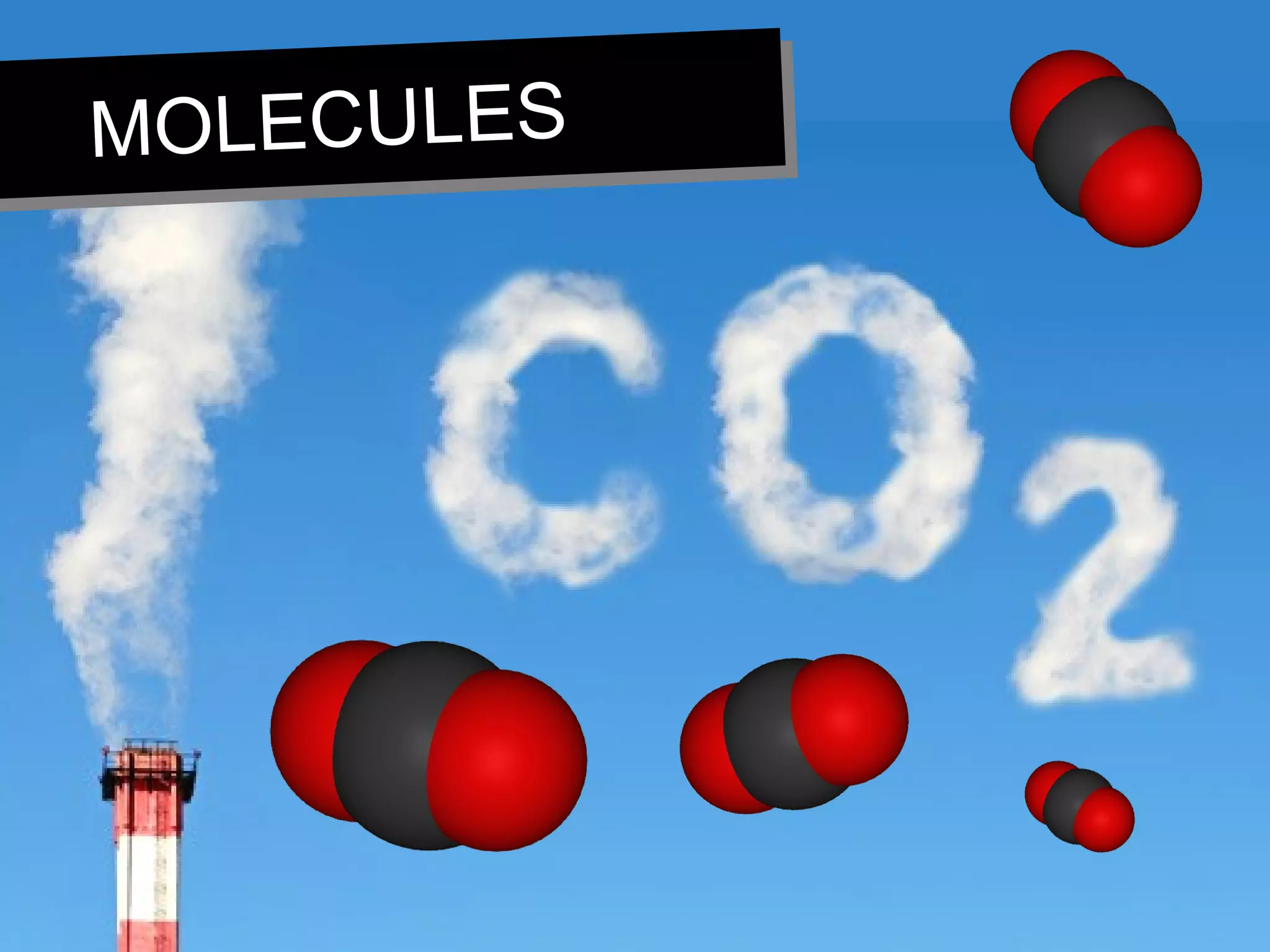
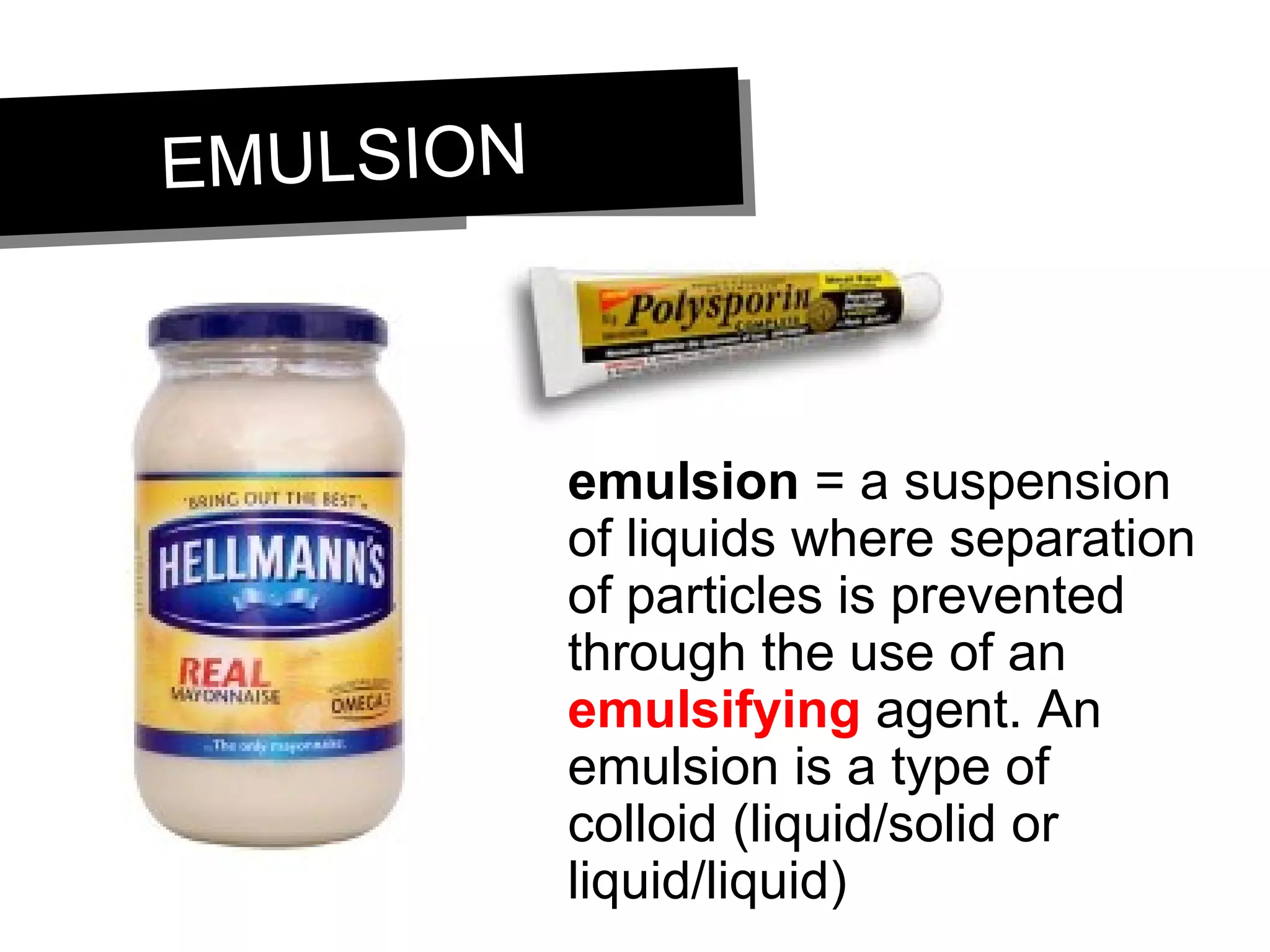
This document discusses the classification of matter. It defines pure substances as substances with constant composition and properties, including elements and compounds. Elements are pure substances that cannot be broken down, while compounds contain two or more elements chemically bonded together. Mixtures contain two or more pure substances that are not chemically combined and can be separated by physical means. Mixtures include homogeneous solutions and heterogeneous mixtures. Homogeneous solutions appear uniform while heterogeneous mixtures contain identifiable components in two or more phases.