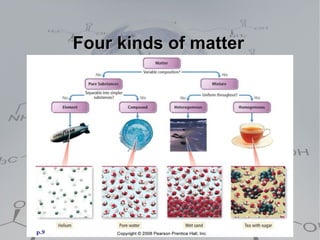
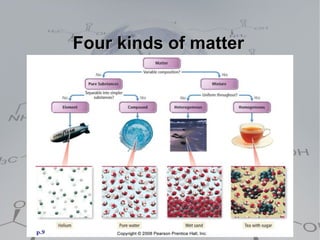
There are four main types of matter:
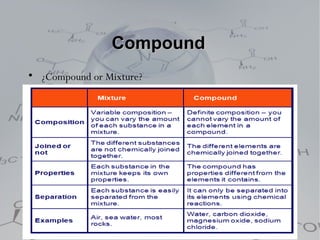
1. Pure substances which are either elements or compounds. Elements cannot be broken down further while compounds can be broken into simpler substances through chemical reactions.
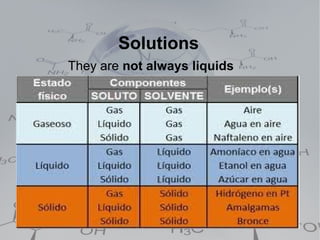
2. Homogeneous mixtures which are uniform throughout and can be either pure substances or solutions. Solutions contain a solute dissolved in a solvent.
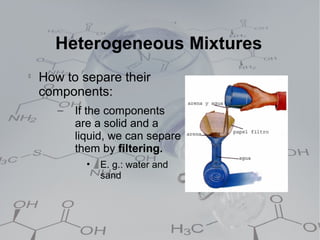
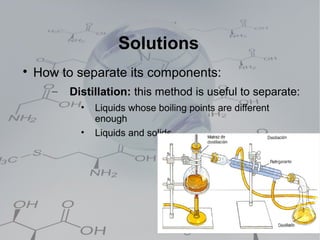
3. Heterogeneous mixtures which are not uniform and have distinct components that can be observed. Components like oil and water or rock components can be separated physically.
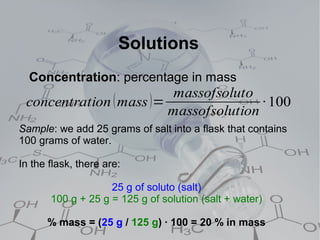
4. Solutions which are homogeneous mixtures with a solute dissolved in a solvent. The concentration of a solution expresses the amount of solute per amount of solution and can be measured in various units.