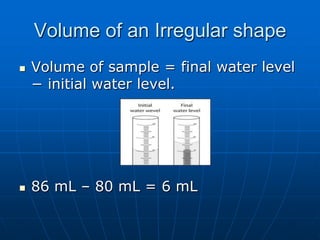
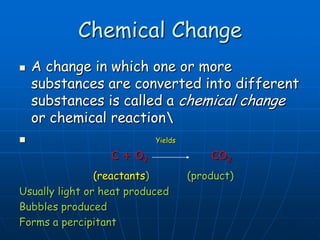
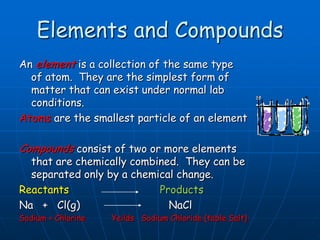
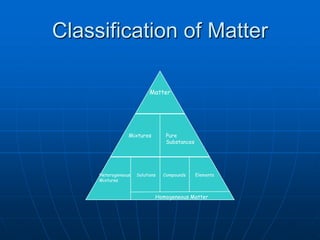
This document defines key concepts about the properties of matter. It explains that matter is anything that has mass and takes up space, and can exist in solid, liquid, or gas states. Physical properties can be observed without changing a substance's identity, while chemical properties involve chemical changes forming new substances. Matter can also be classified as pure substances like elements and compounds, or mixtures that are either homogeneous solutions or heterogeneous mixtures of different phases.