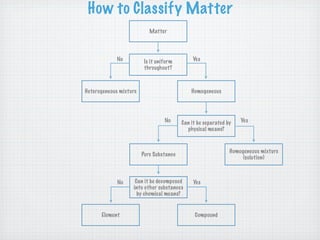
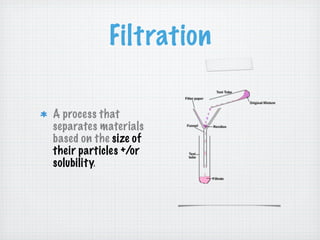
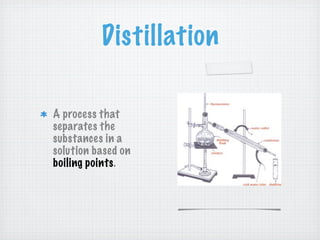
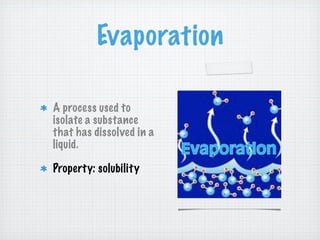
This document discusses different types of matter and changes in matter. It defines key terms like elements, compounds, mixtures, solutions, suspensions, and colloids. It explains that a pure substance has a uniform composition while a mixture's composition can vary. Physical properties can be observed without changing a substance's composition, while chemical properties involve changes in composition. Common separation methods like filtration and distillation are also outlined. The document stresses that physical changes do not alter a substance's composition, while chemical changes produce new substances.