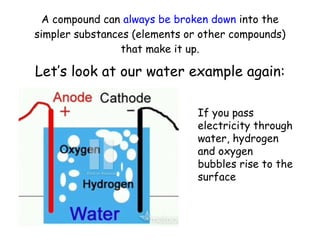
This document discusses the classification of matter into pure substances and mixtures. Pure substances are either elements or compounds, with elements made of only one type of atom and compounds made by the chemical combination of elements. Compounds can be broken down into their constituent elements or other compounds. Mixtures contain two or more substances mixed together without chemical bonding, and can be either heterogeneous, with noticeably different parts, or homogeneous, with an even distribution making the parts indistinguishable.