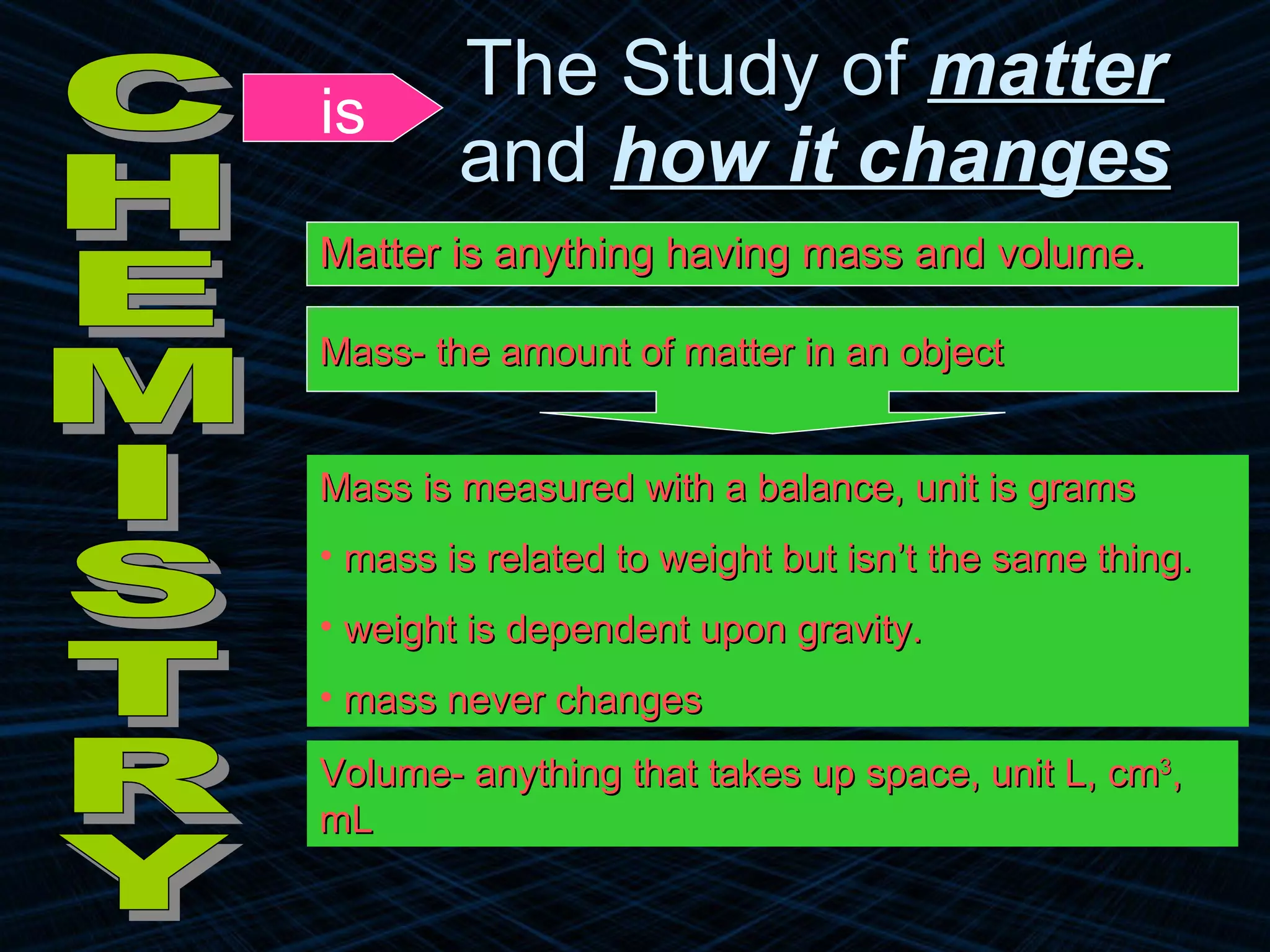
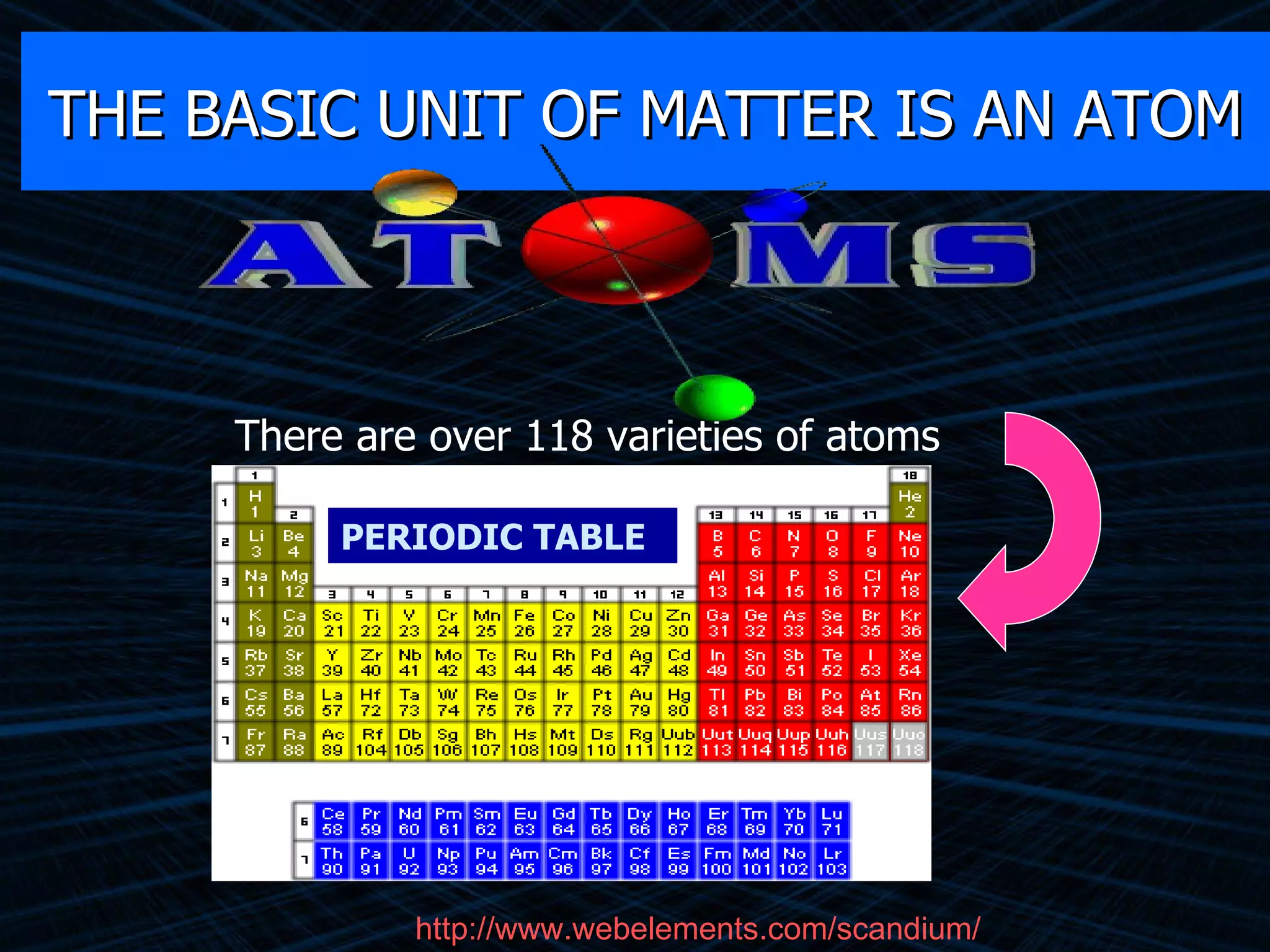
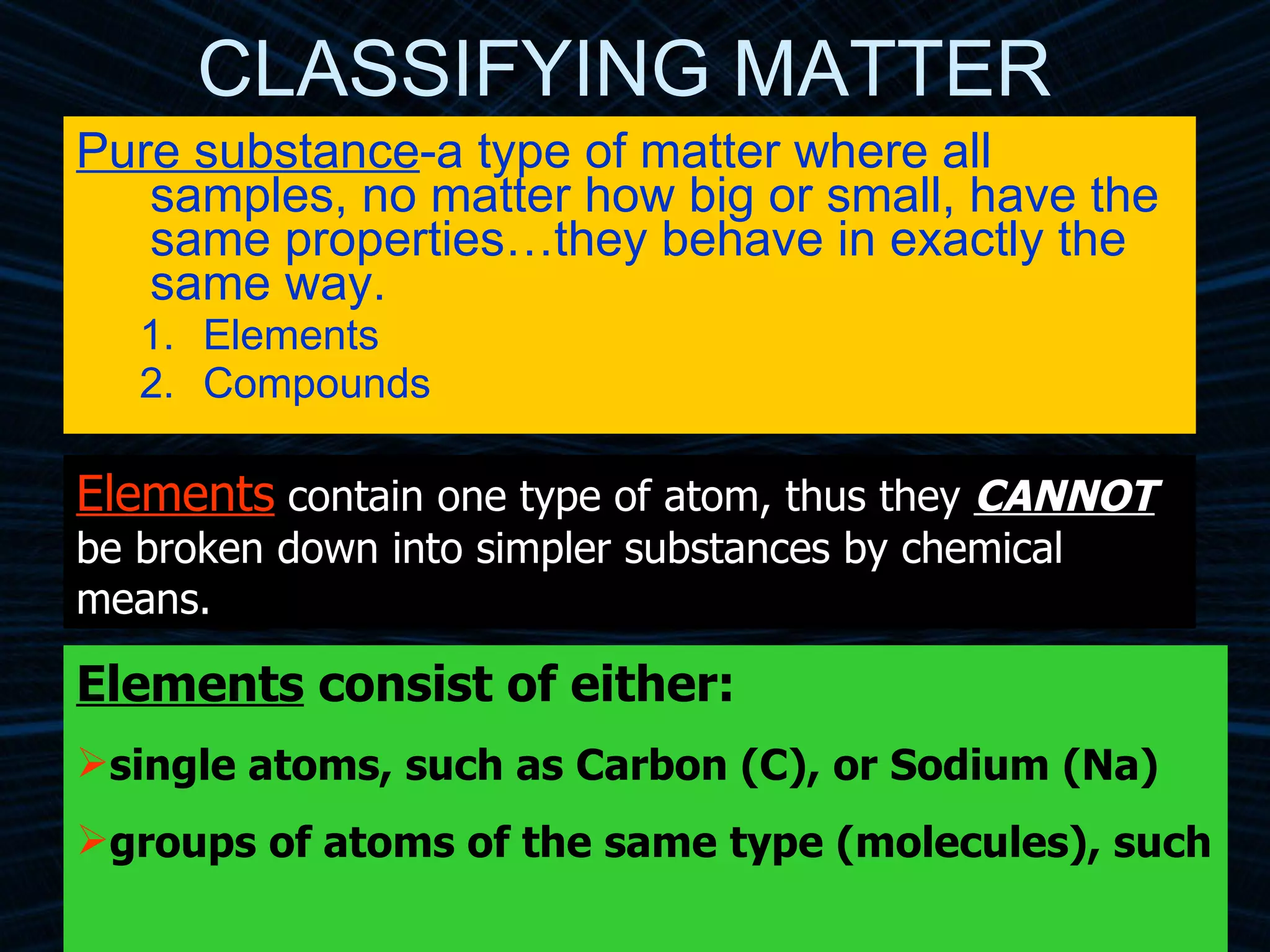
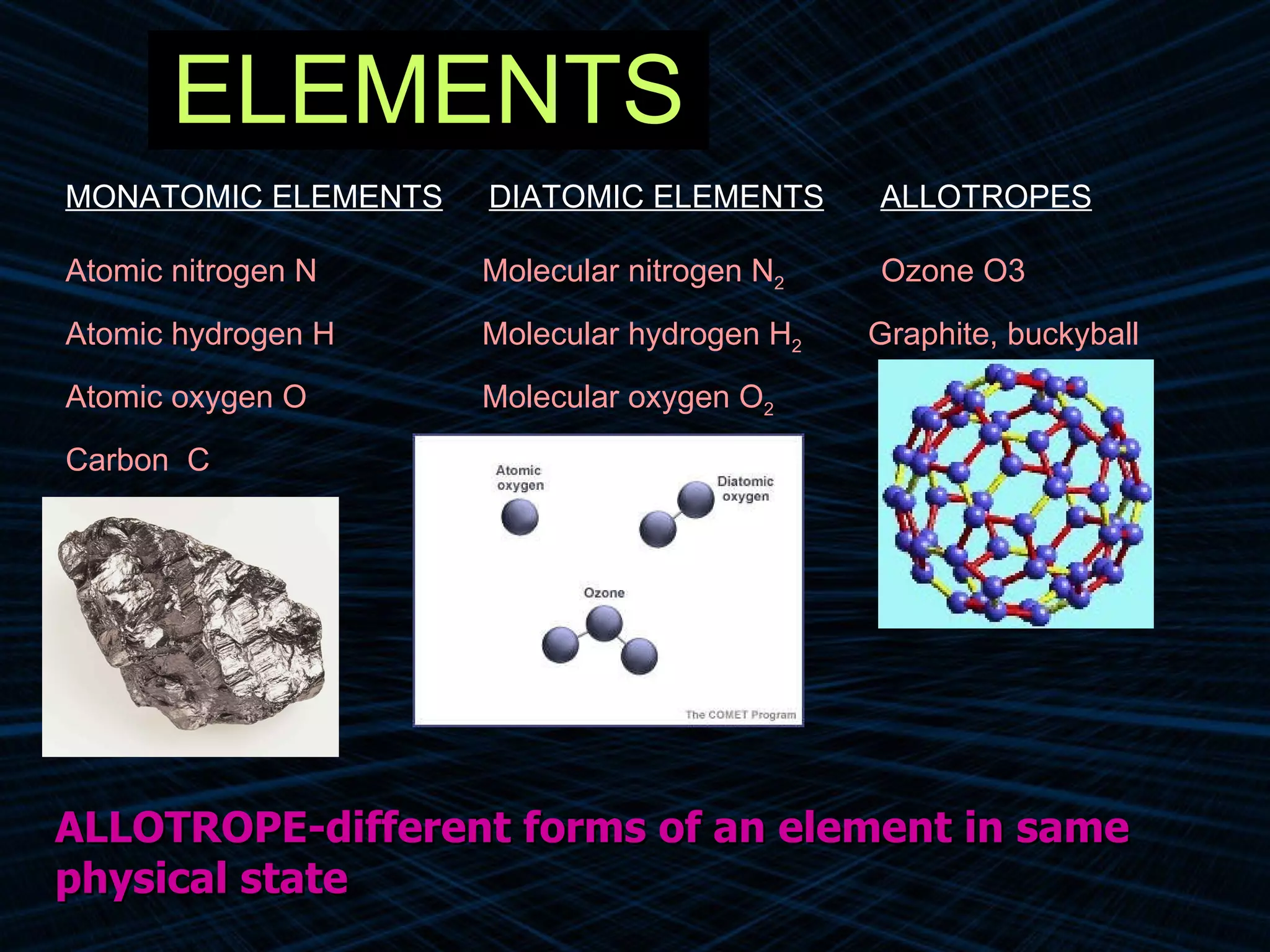
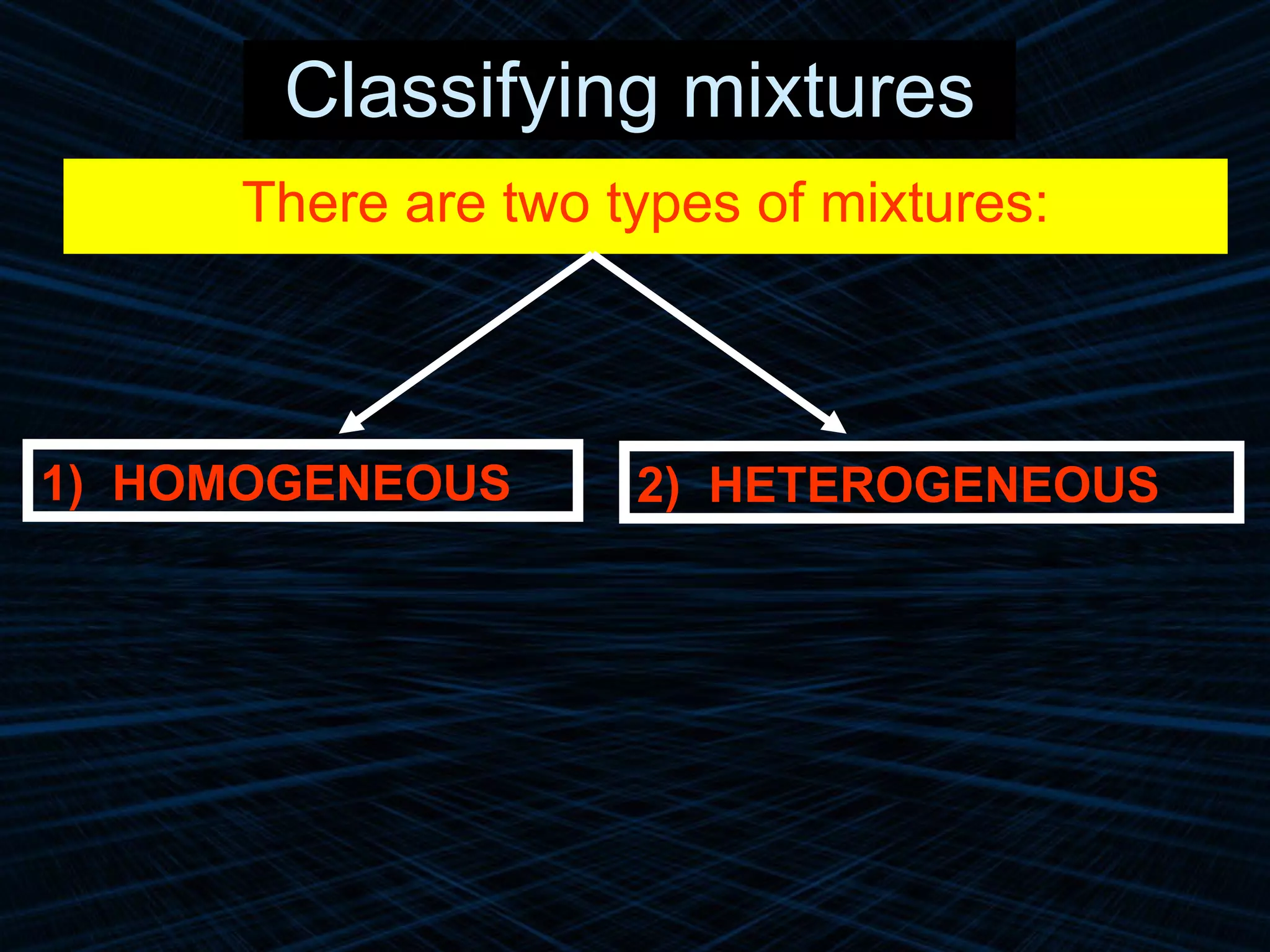
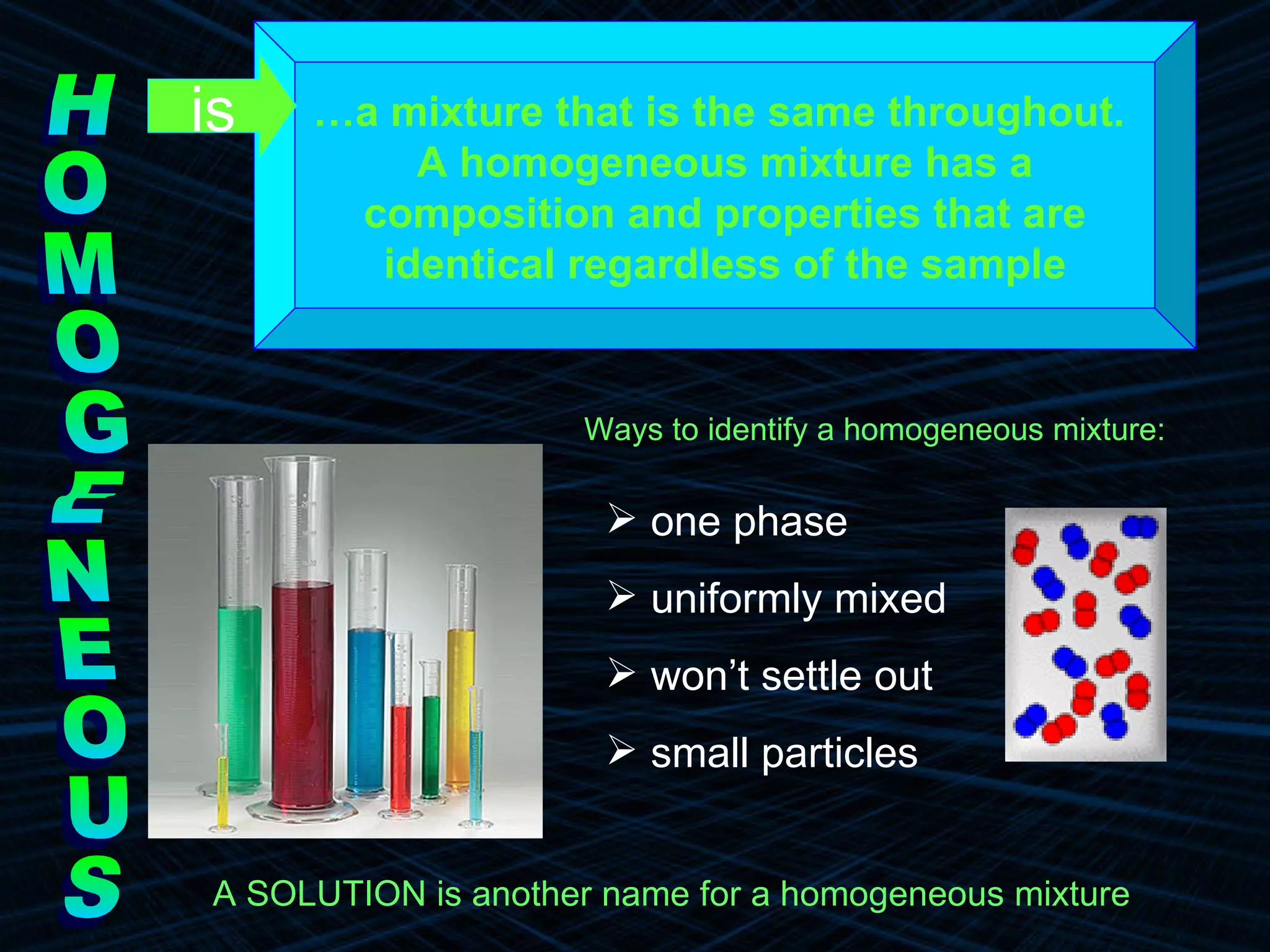
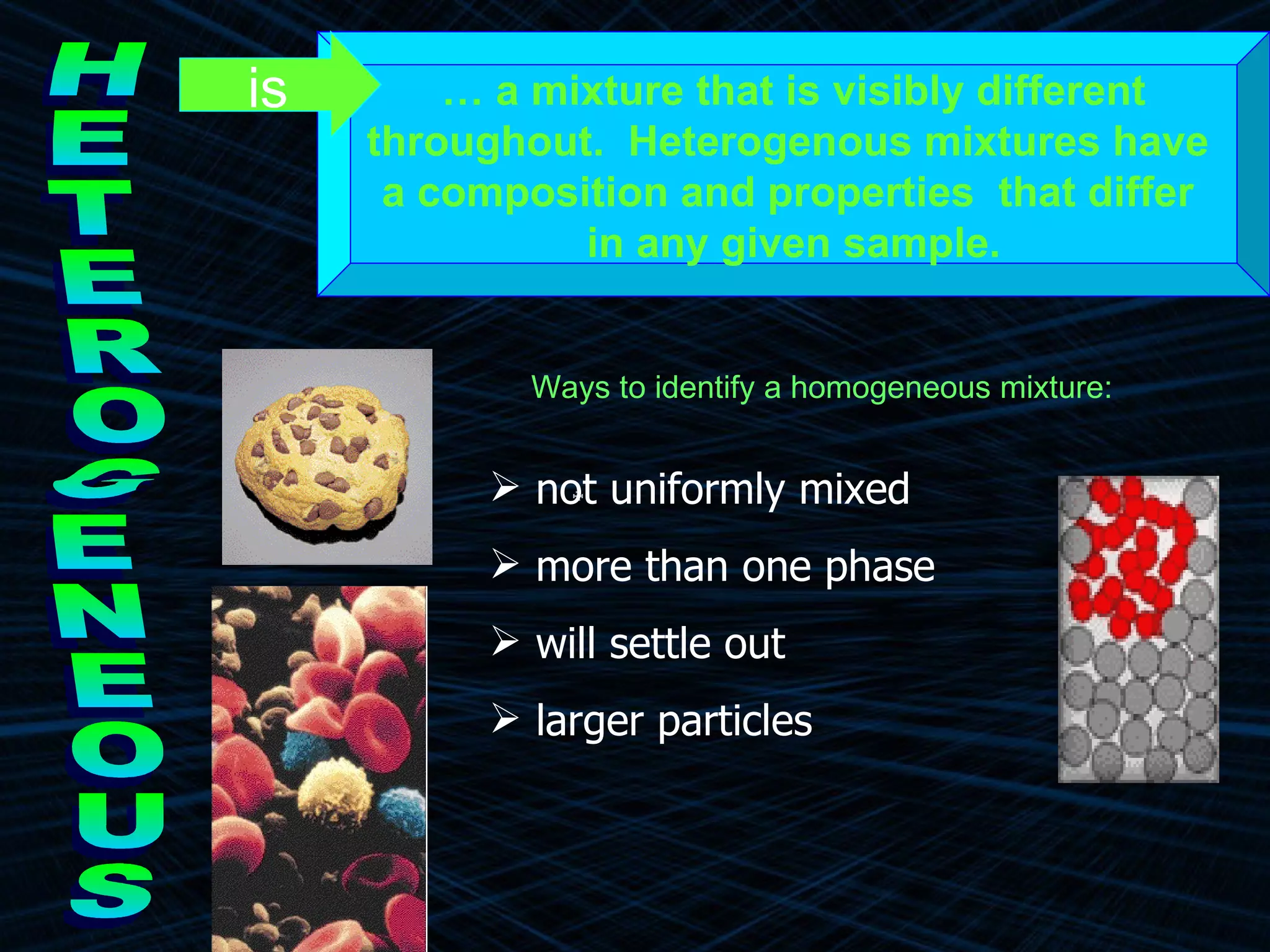
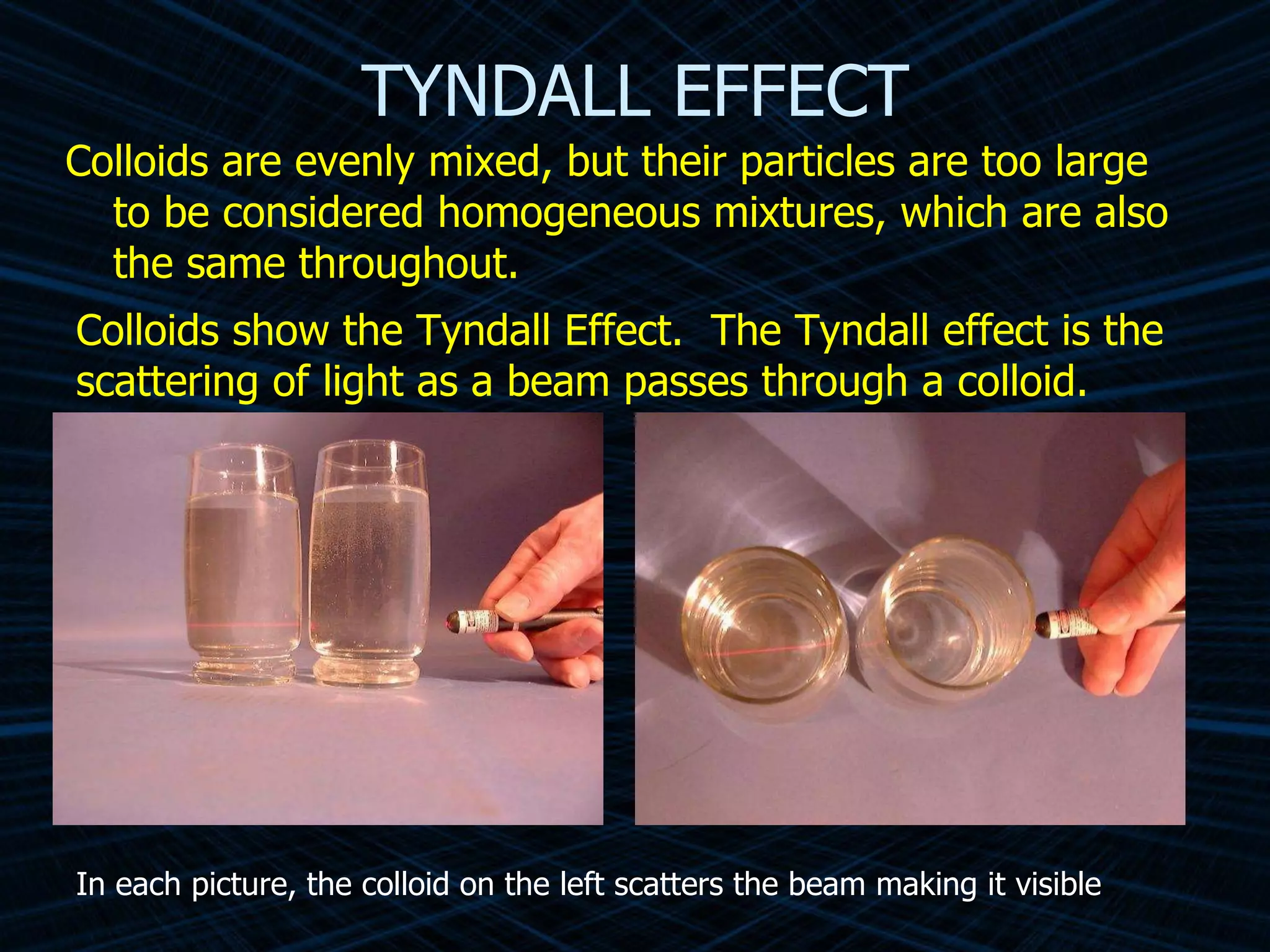
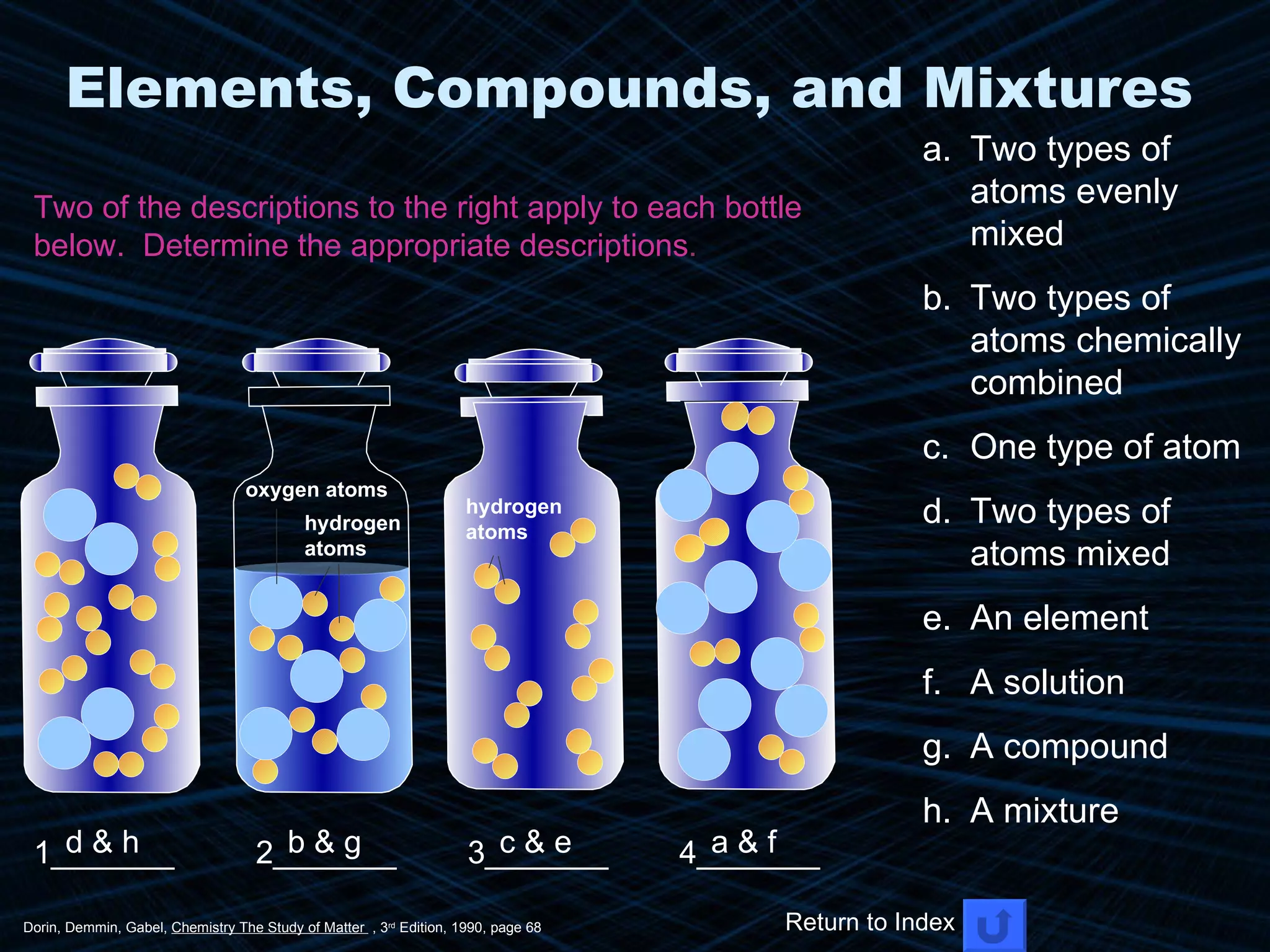
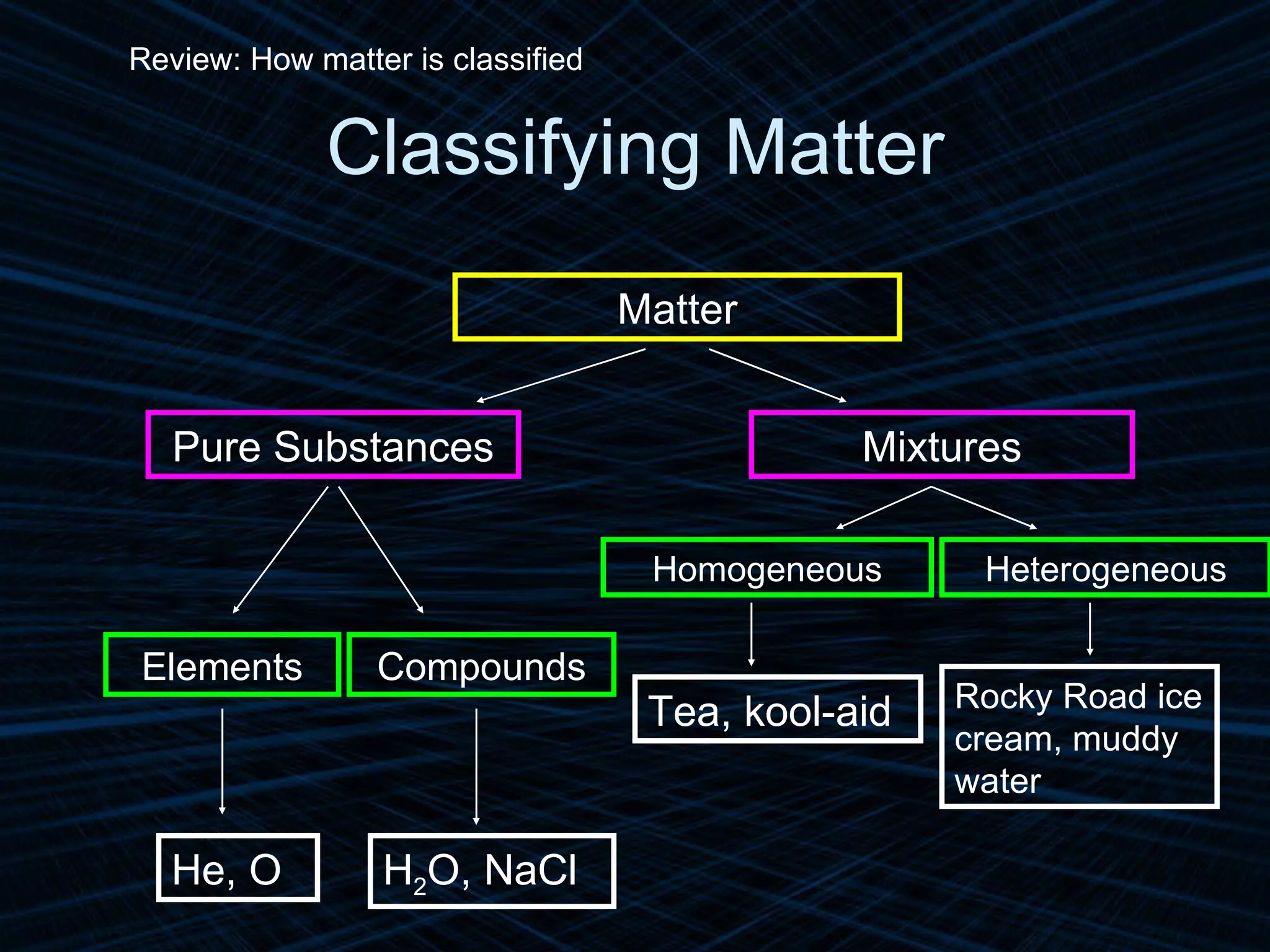
The document provides an overview of classifying and studying matter. It defines matter as anything having mass and volume. It discusses the basic units of matter being atoms and classifies matter as either pure substances (elements or compounds) or mixtures. Elements contain only one type of atom, while compounds are made of two or more different elements that are chemically combined. Mixtures are combinations of substances that are not chemically combined and can be separated physically. Mixtures are either homogeneous, appearing uniform throughout, or heterogeneous, visibly different throughout. Examples and diagrams are provided to illustrate these key concepts.