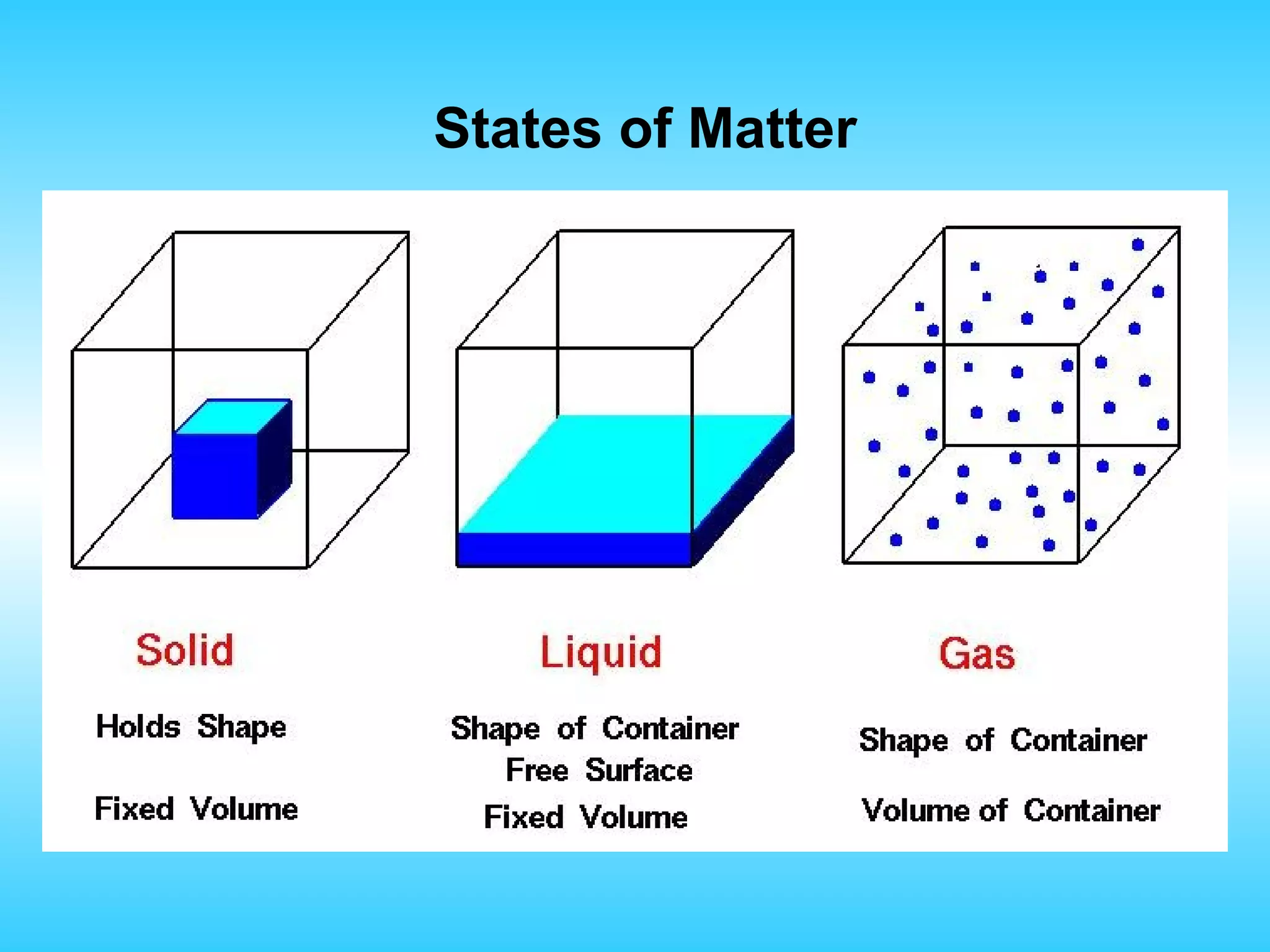
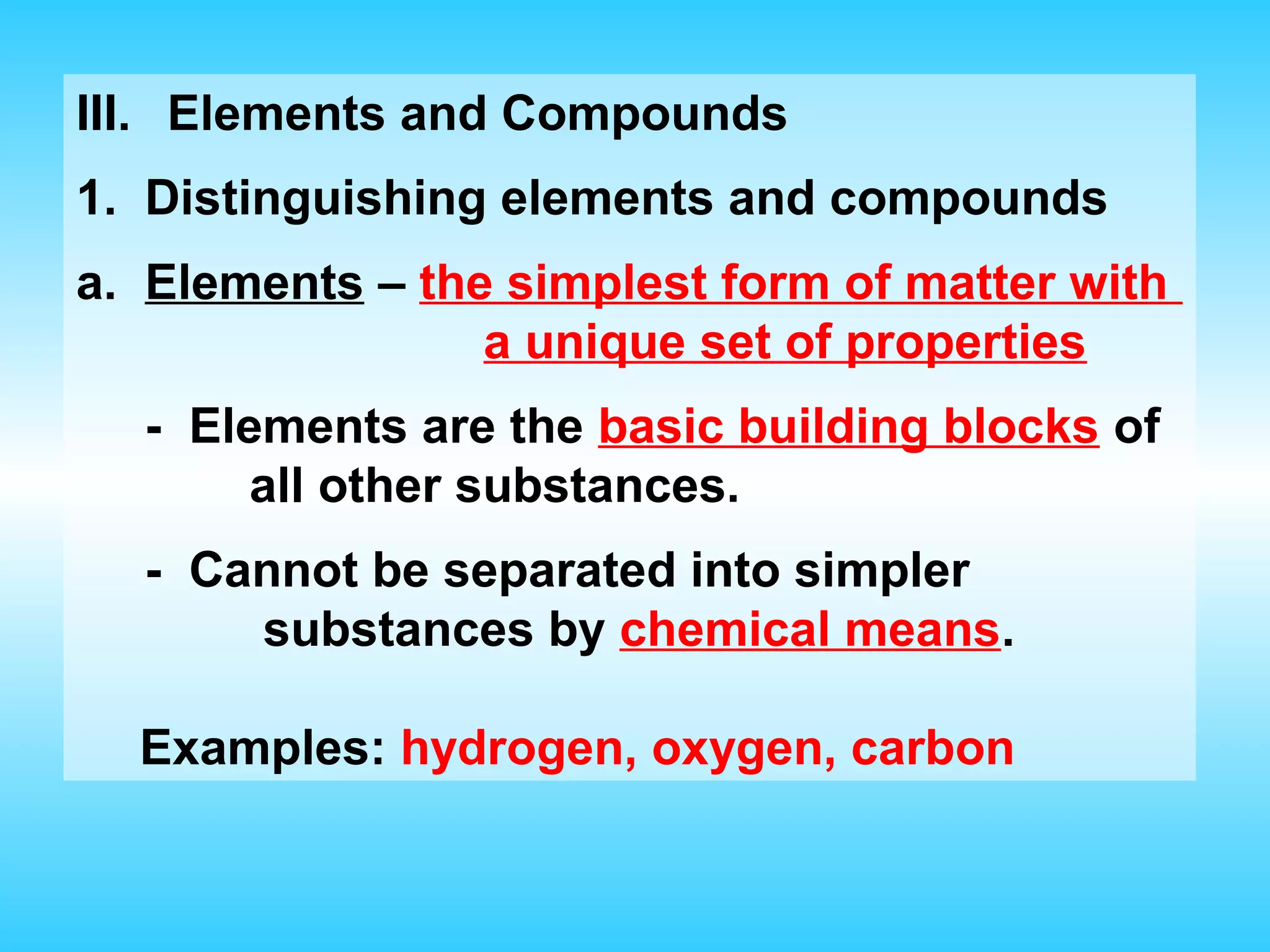
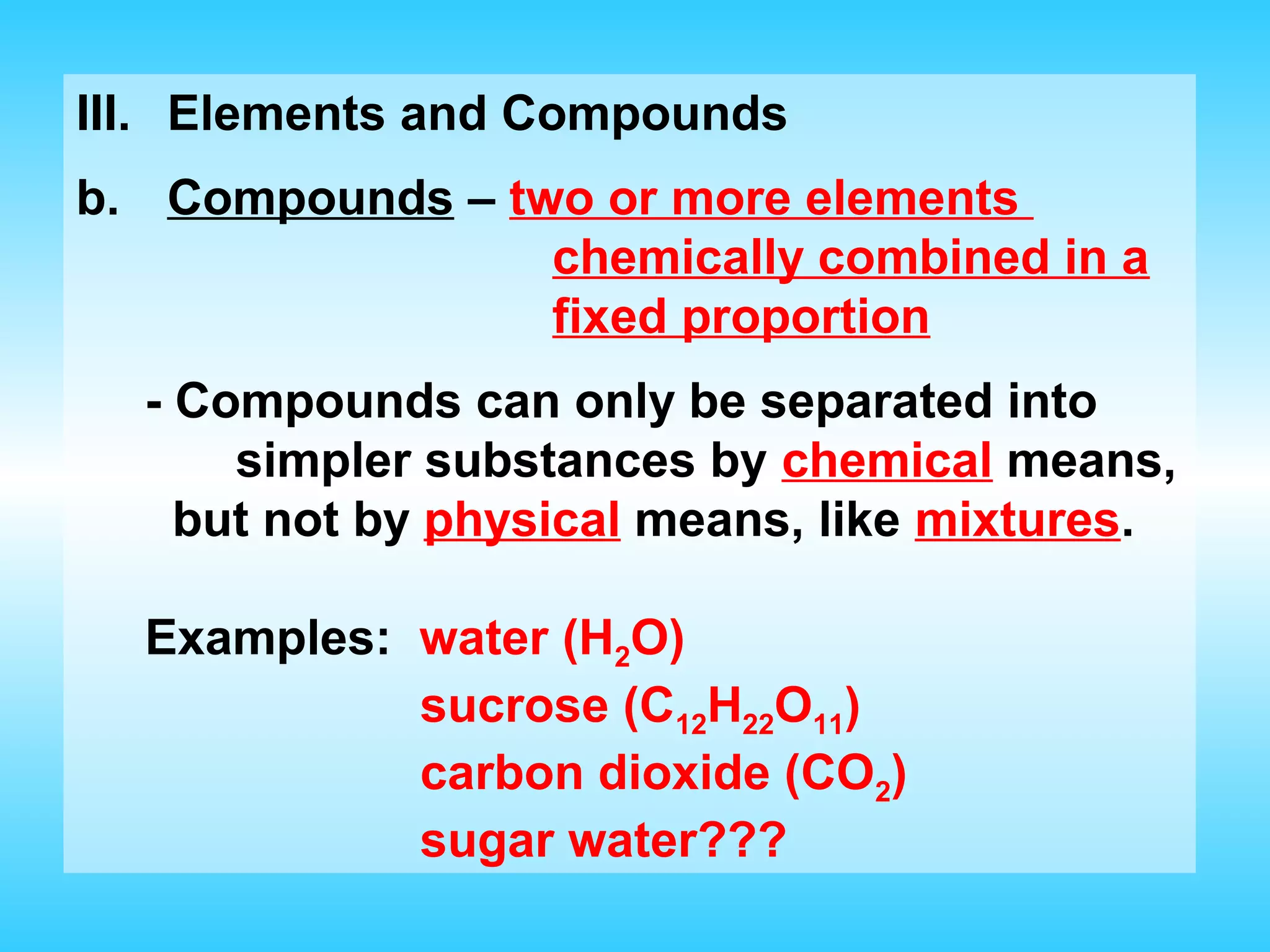
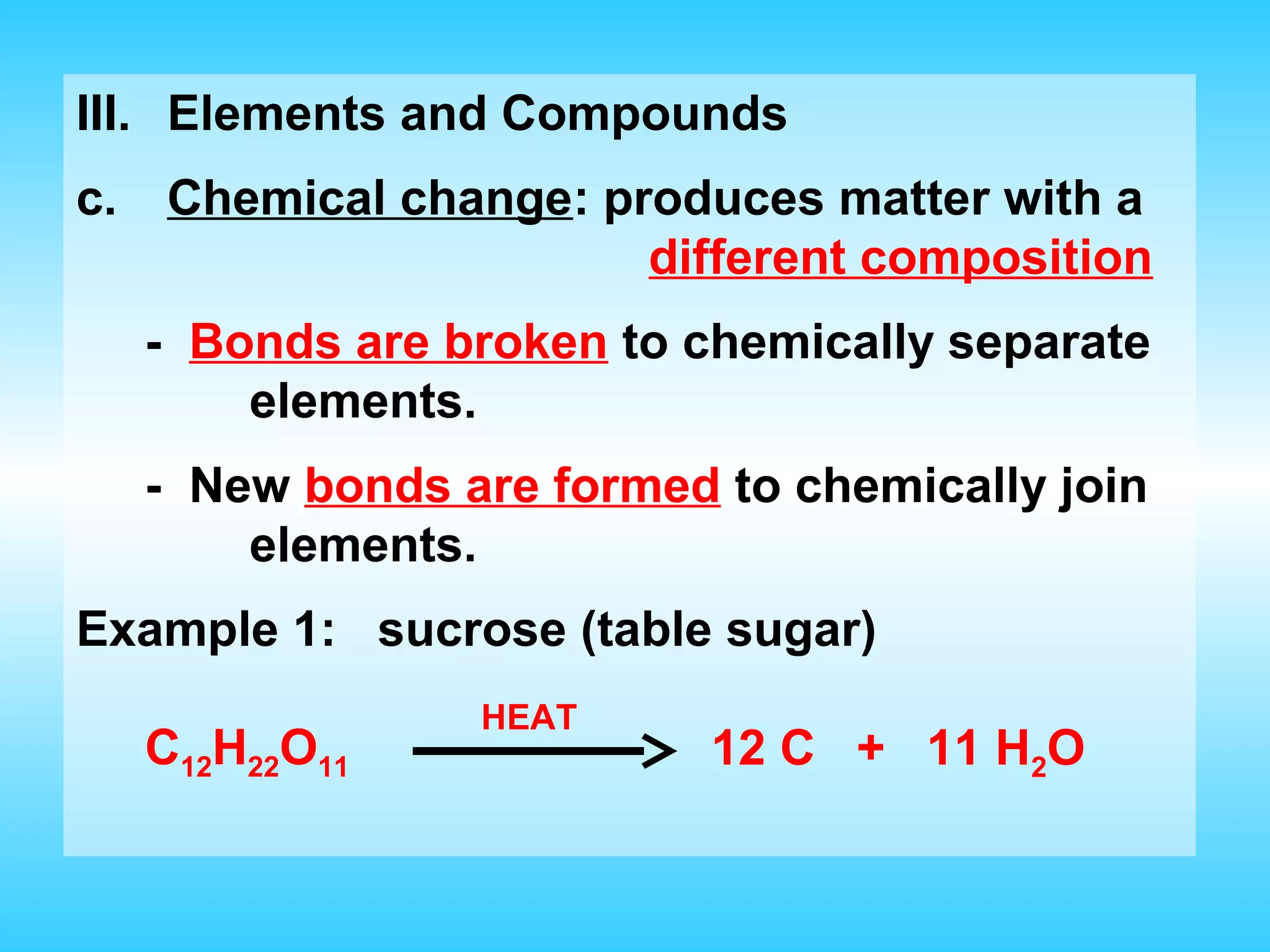
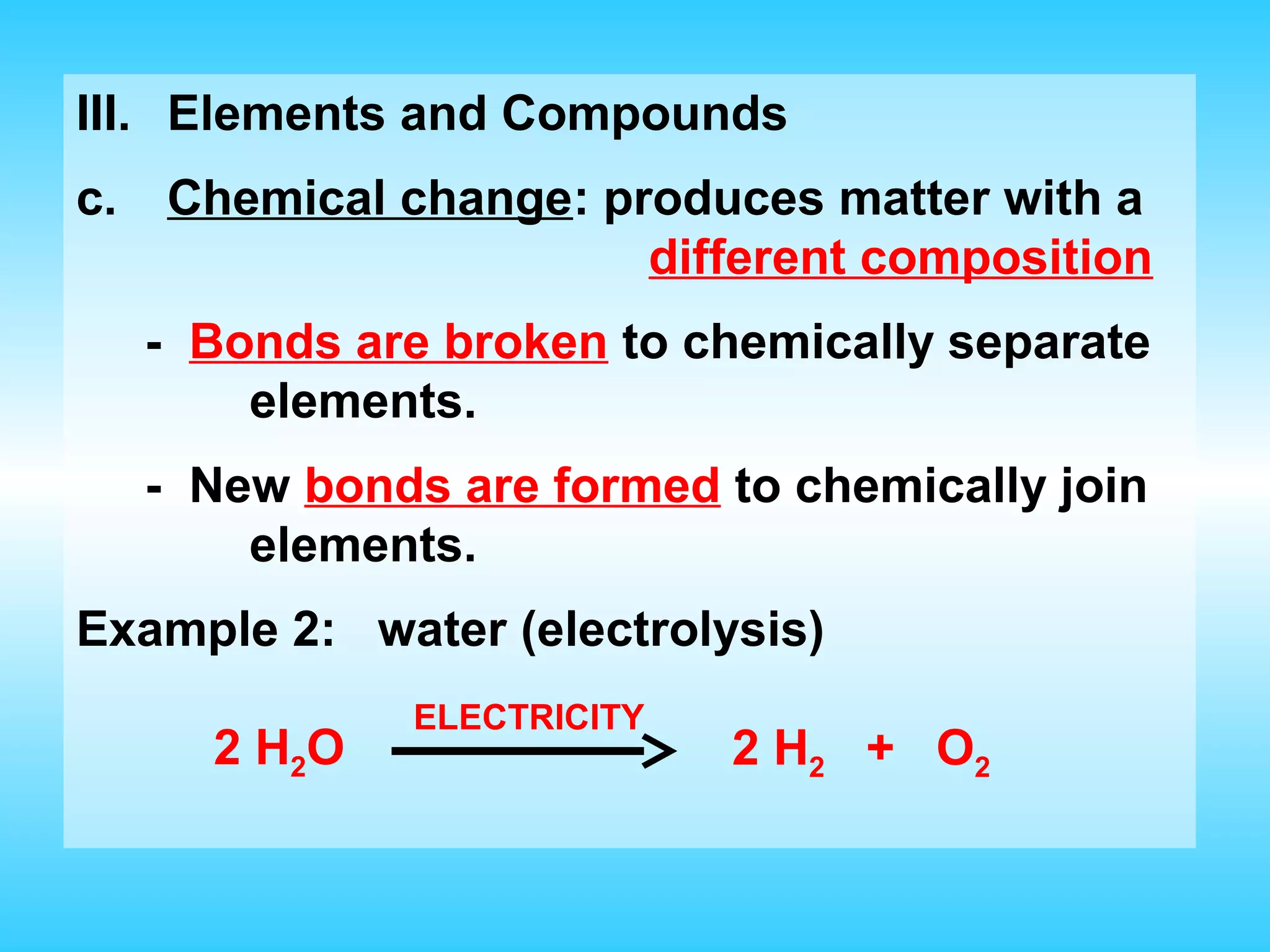
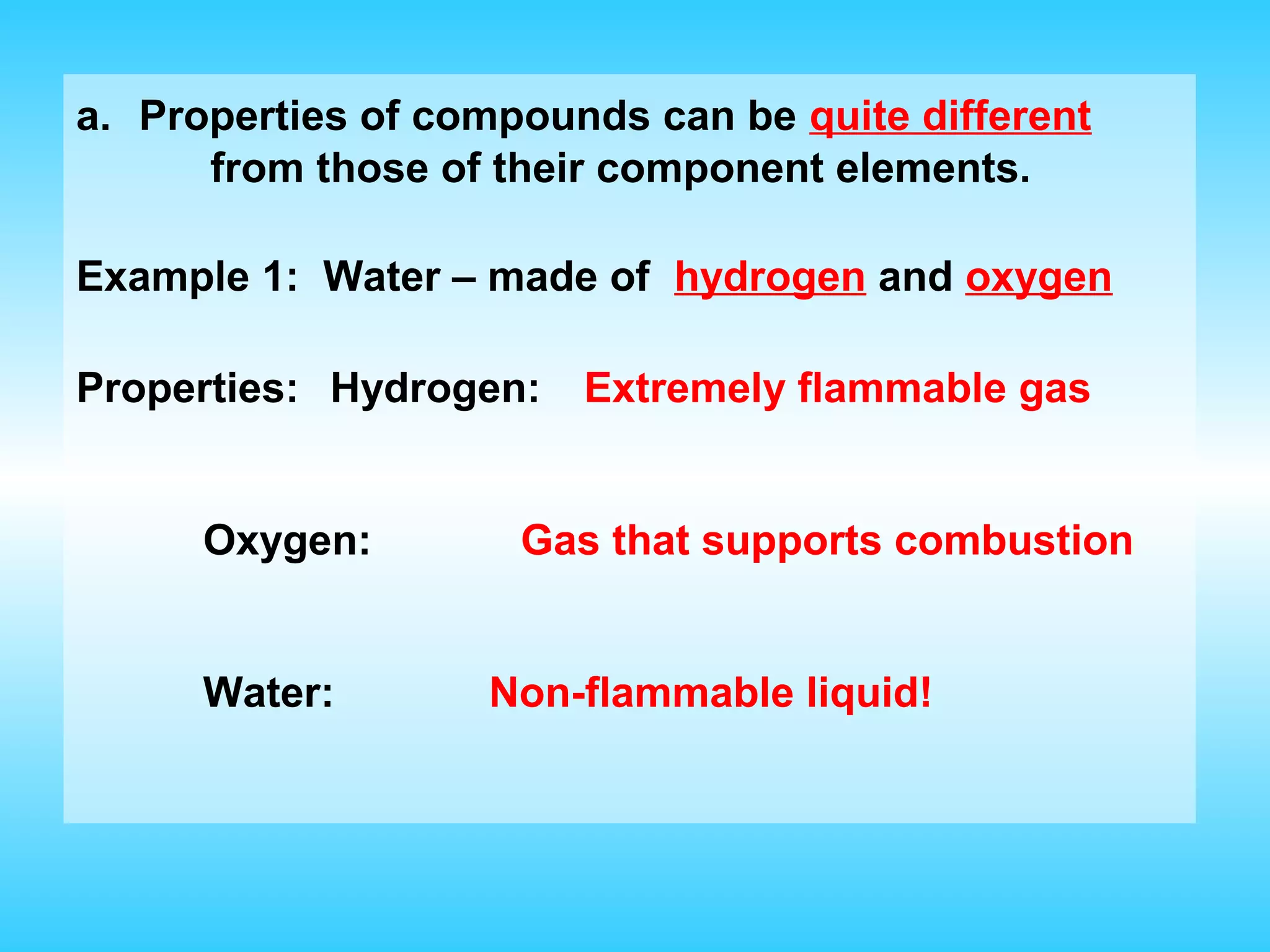
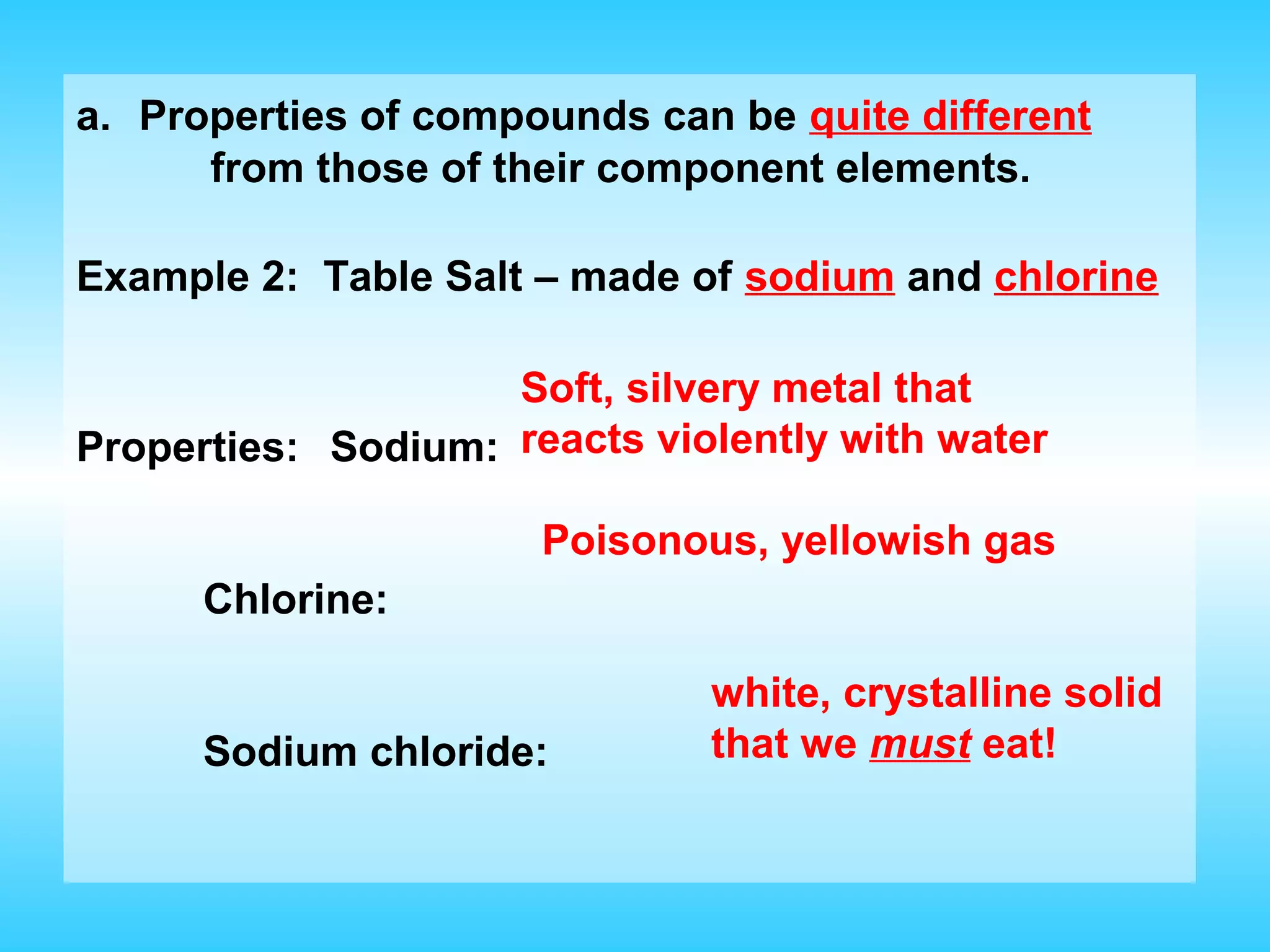
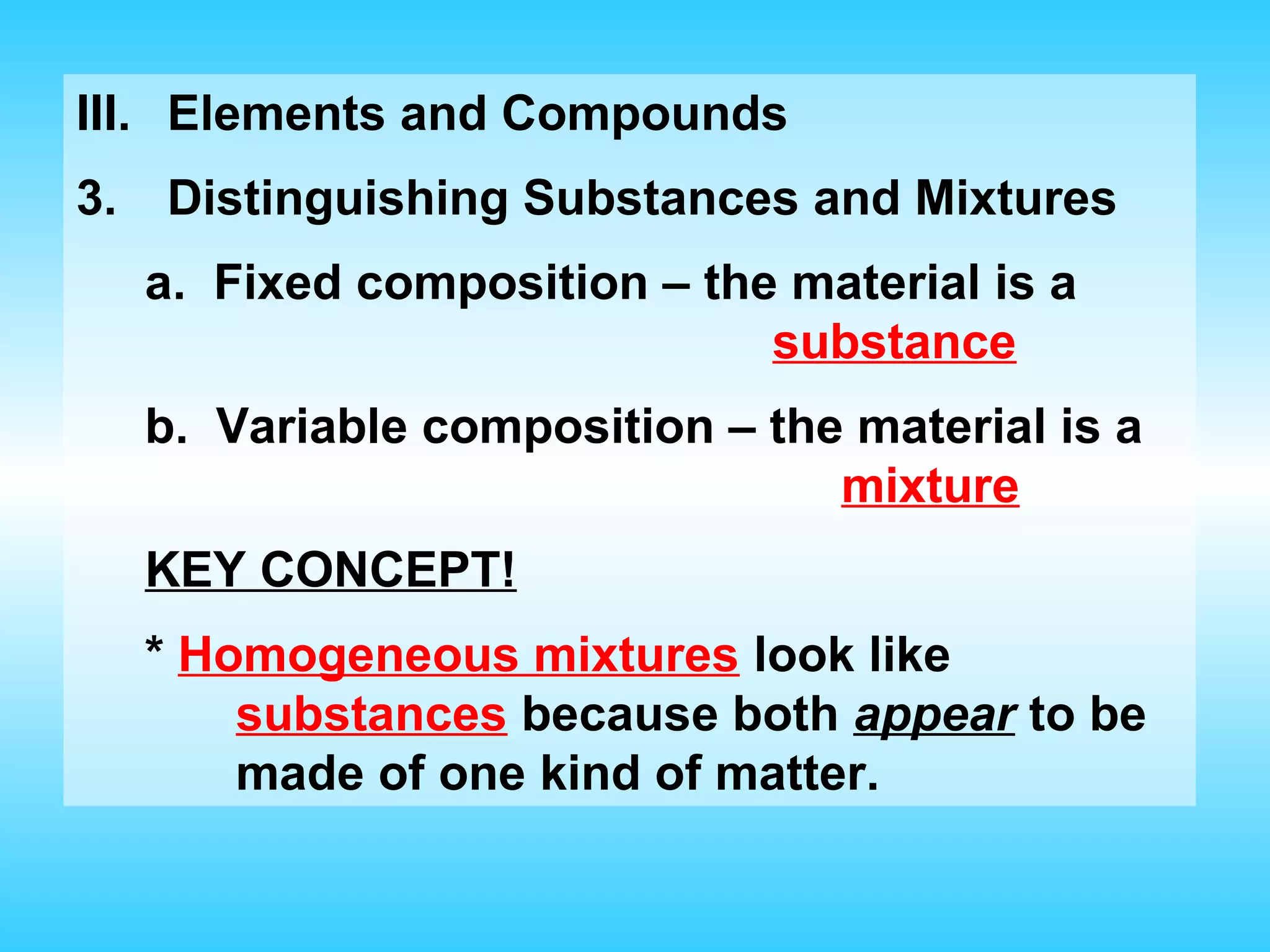
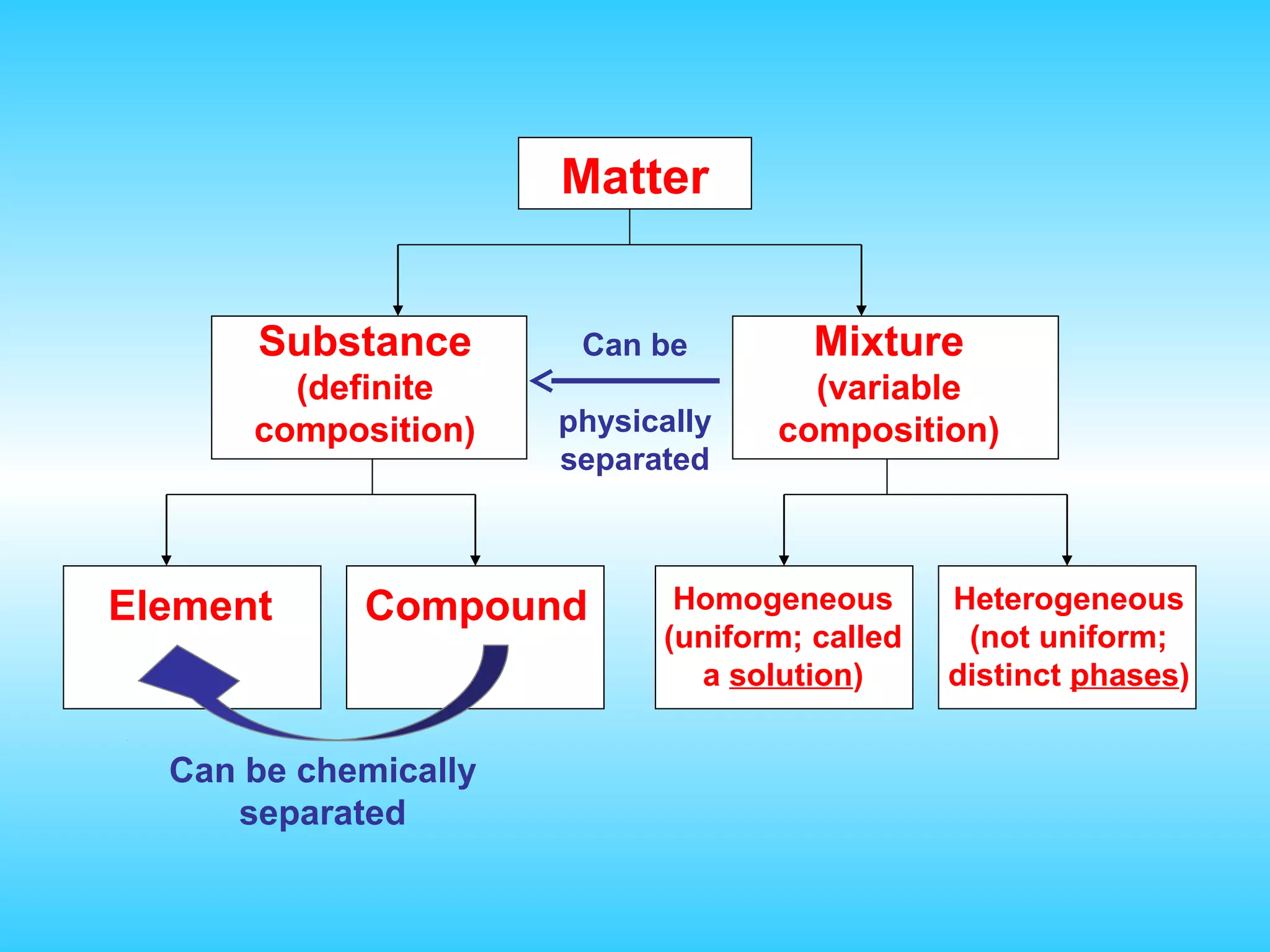
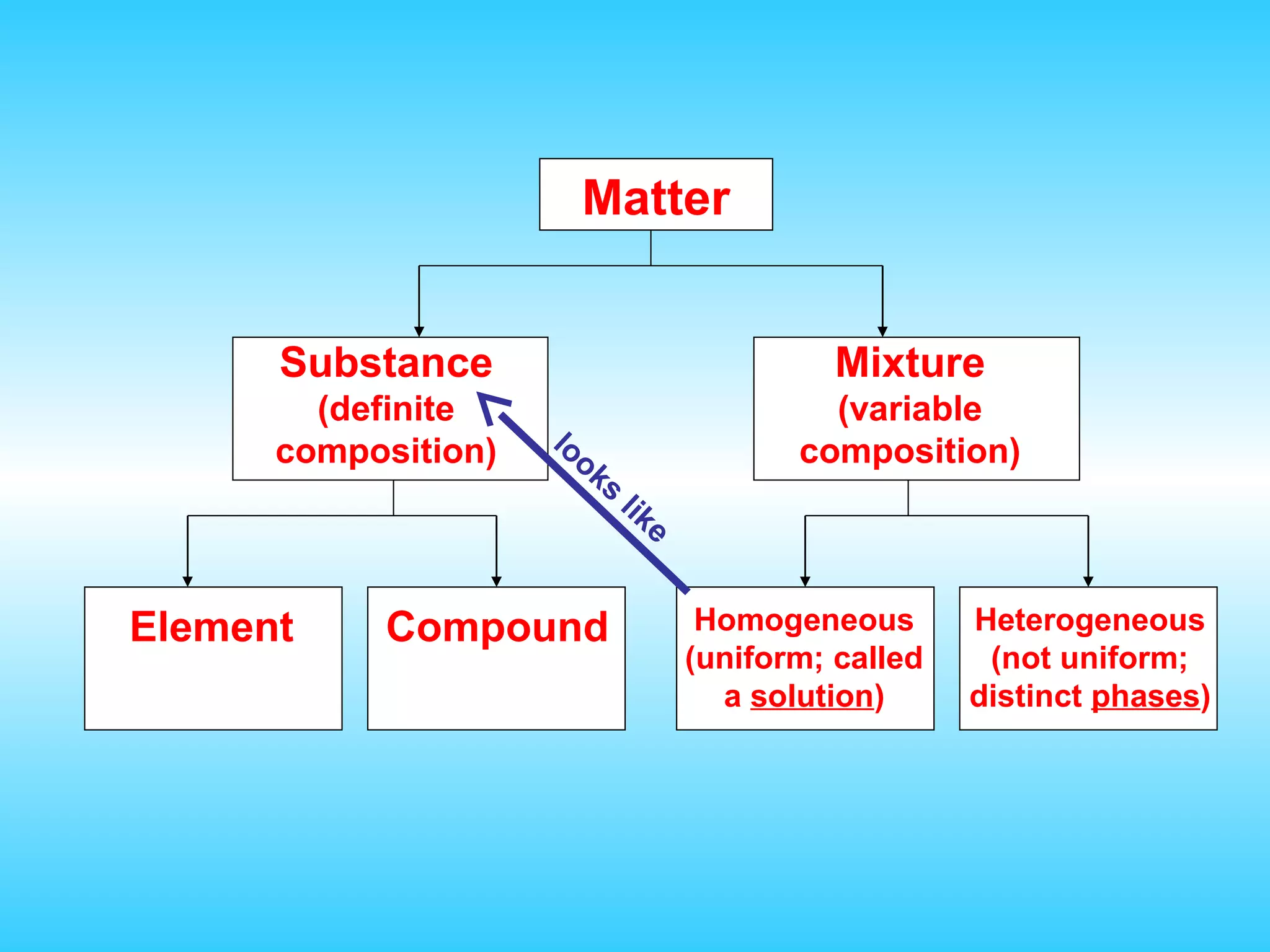
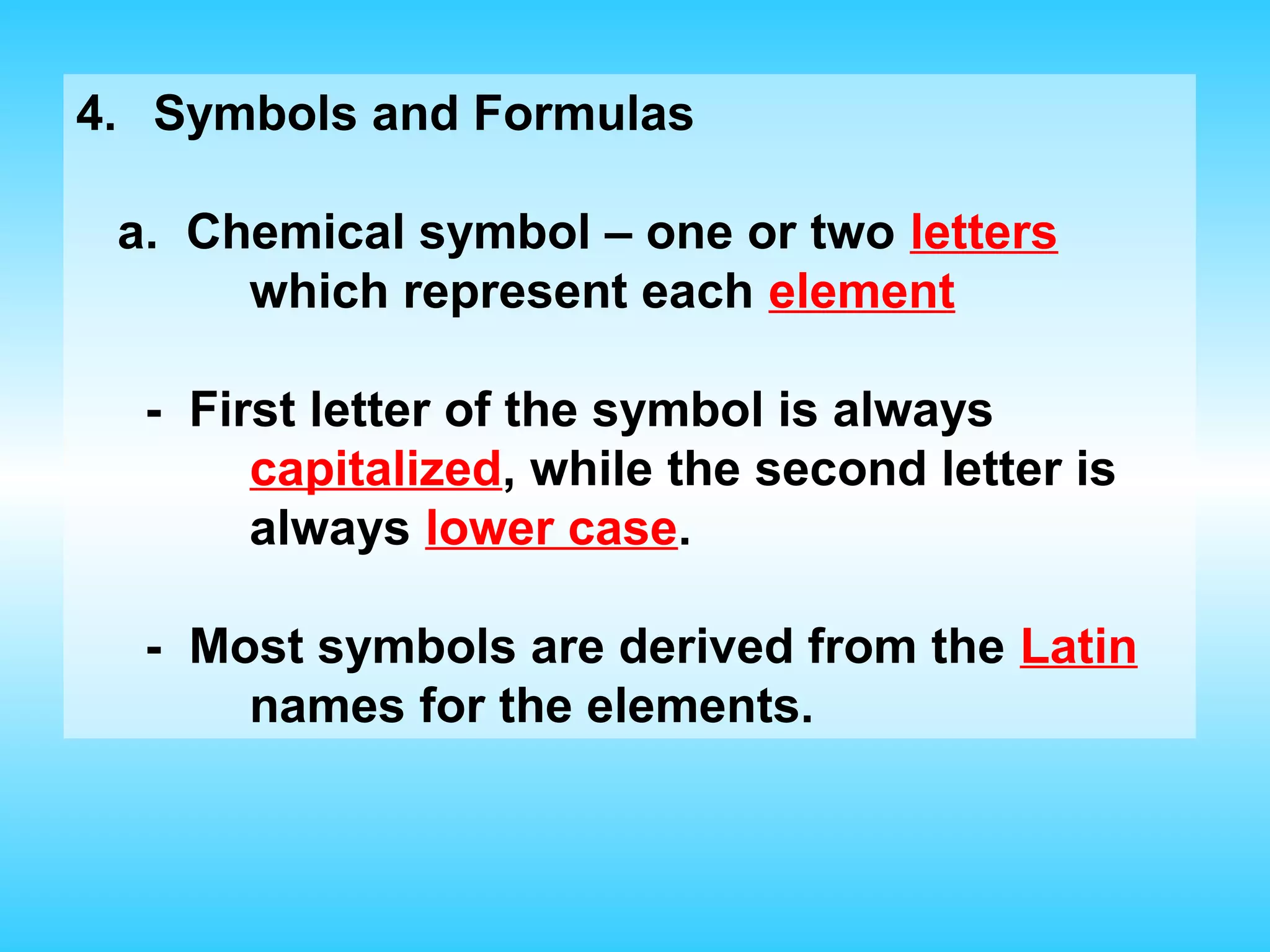
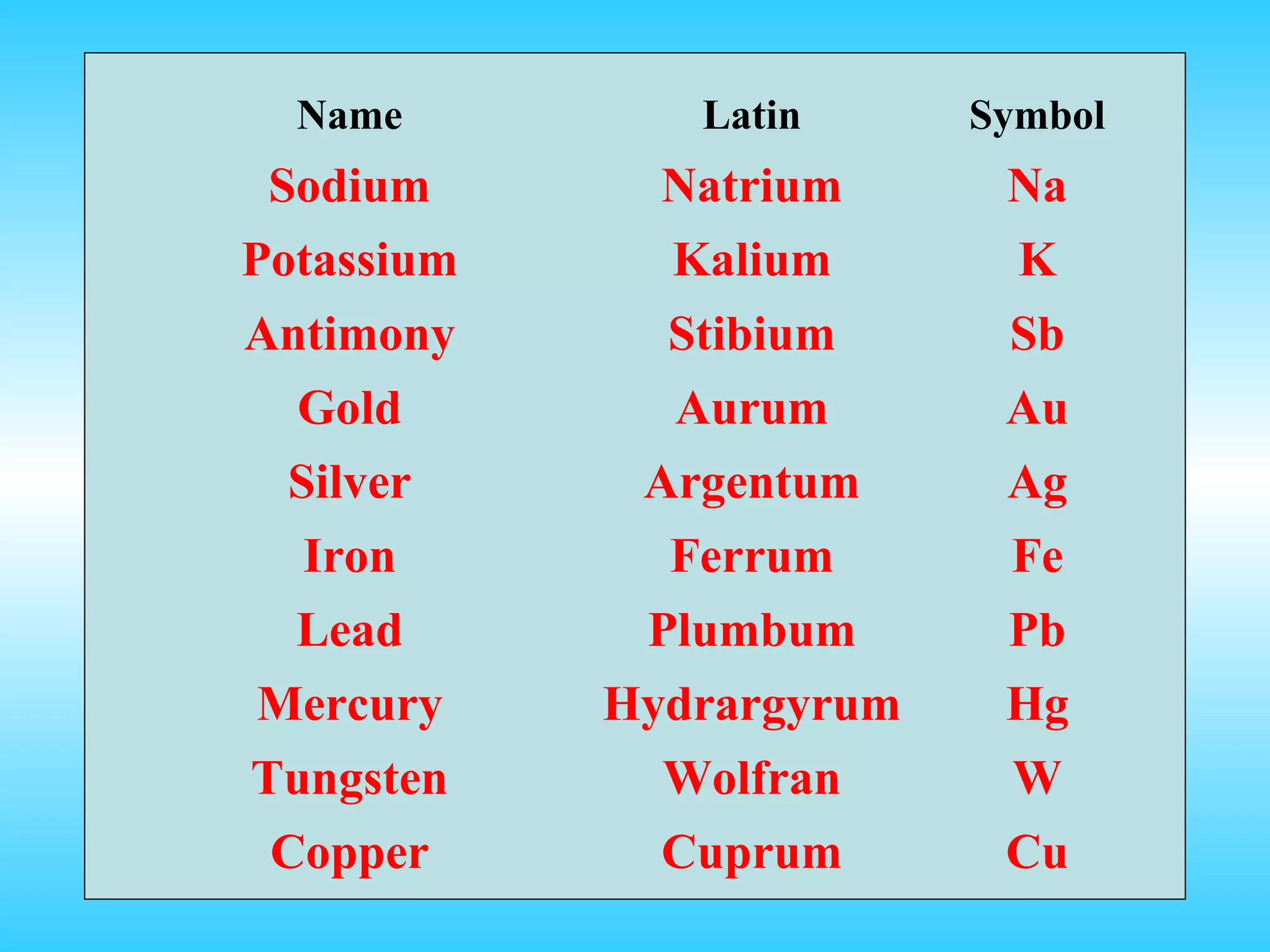
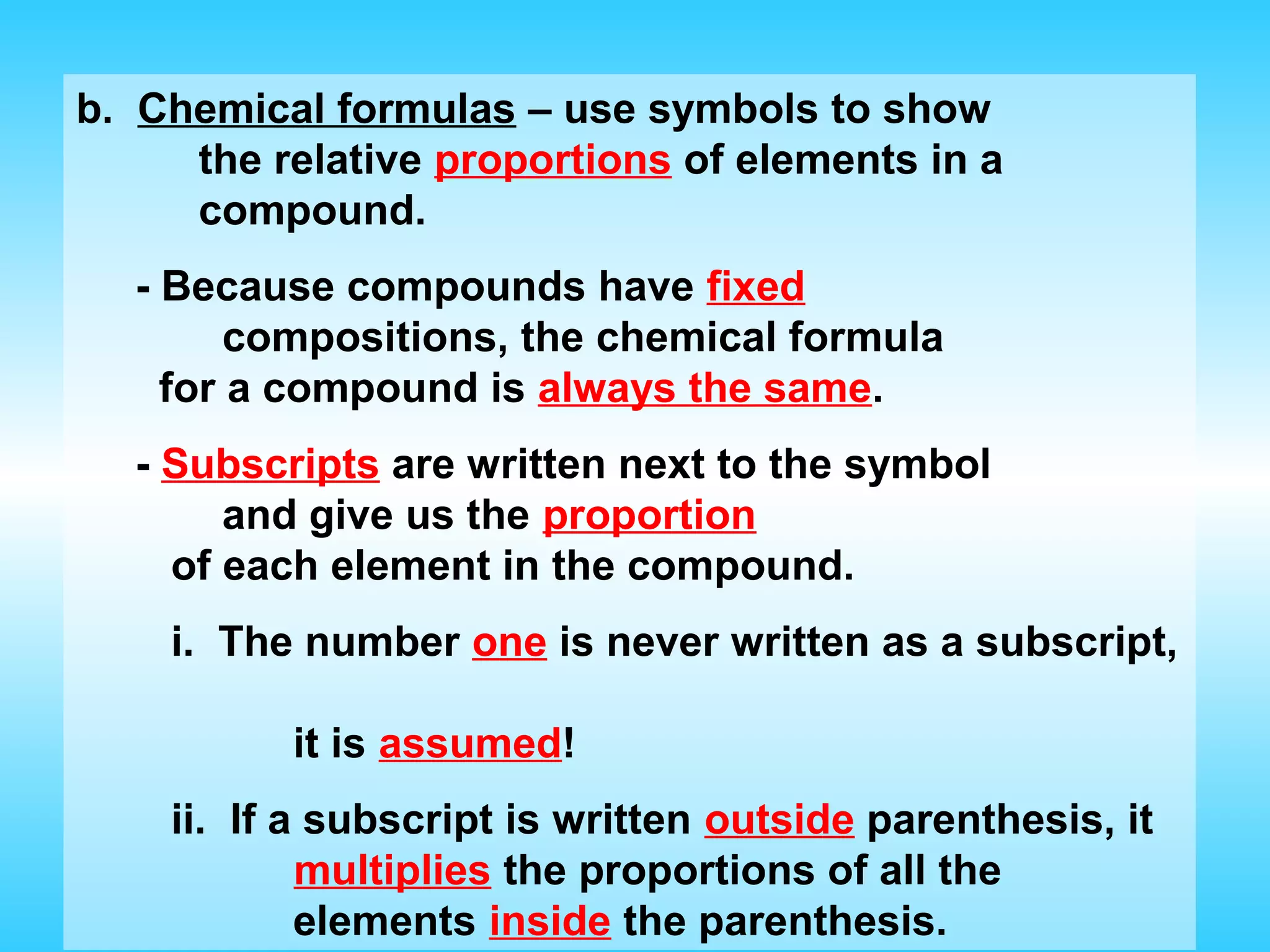
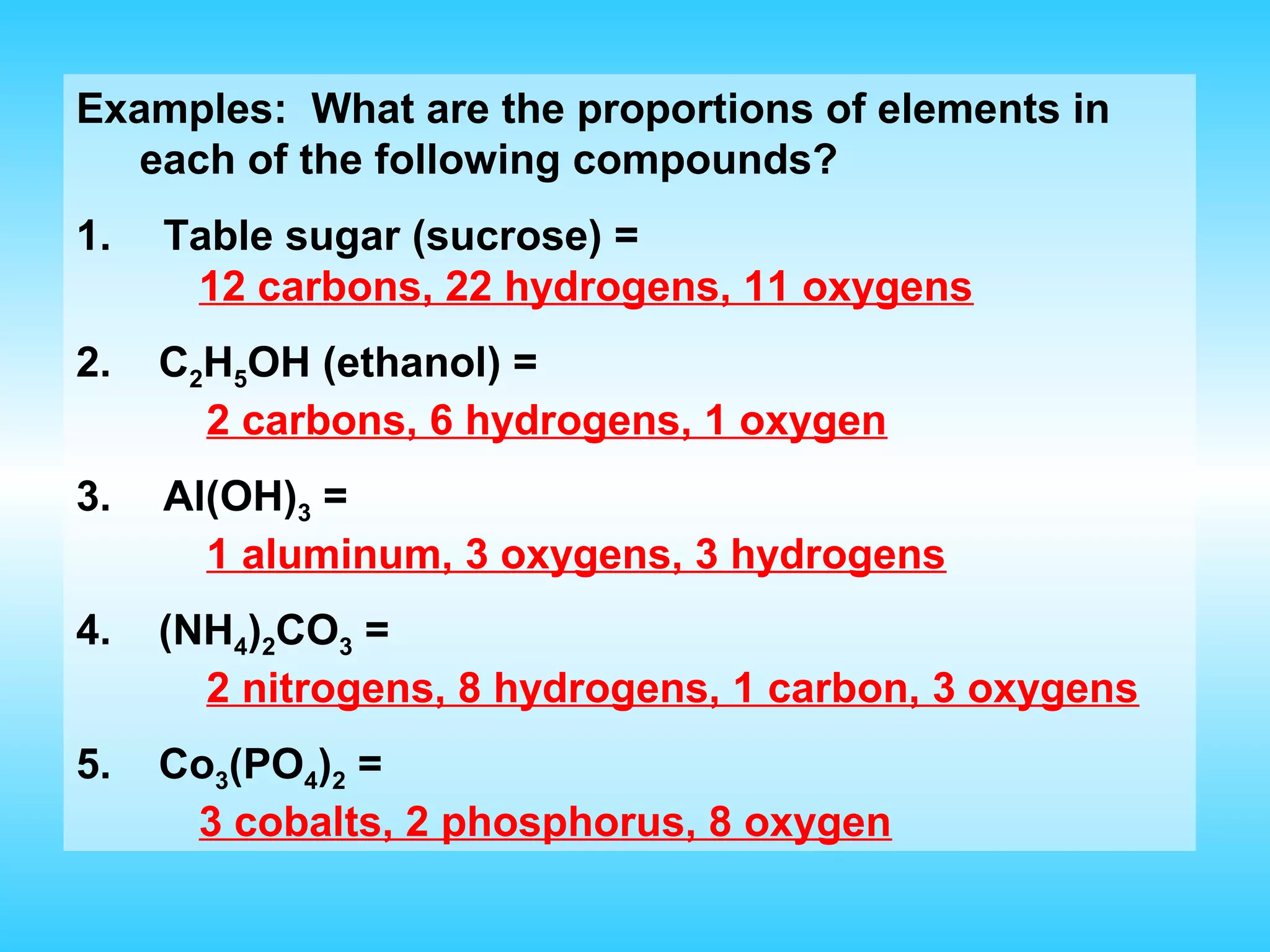
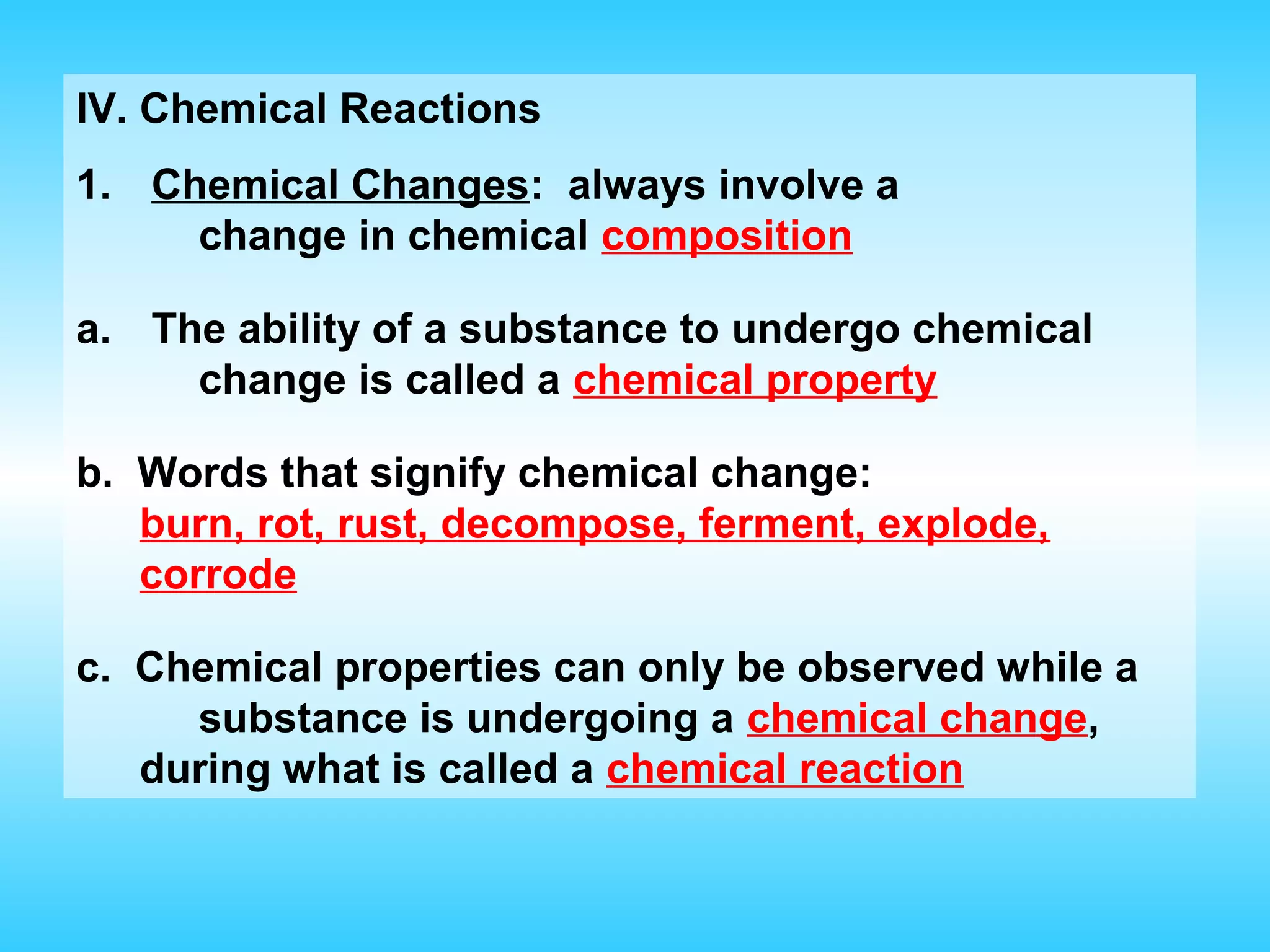
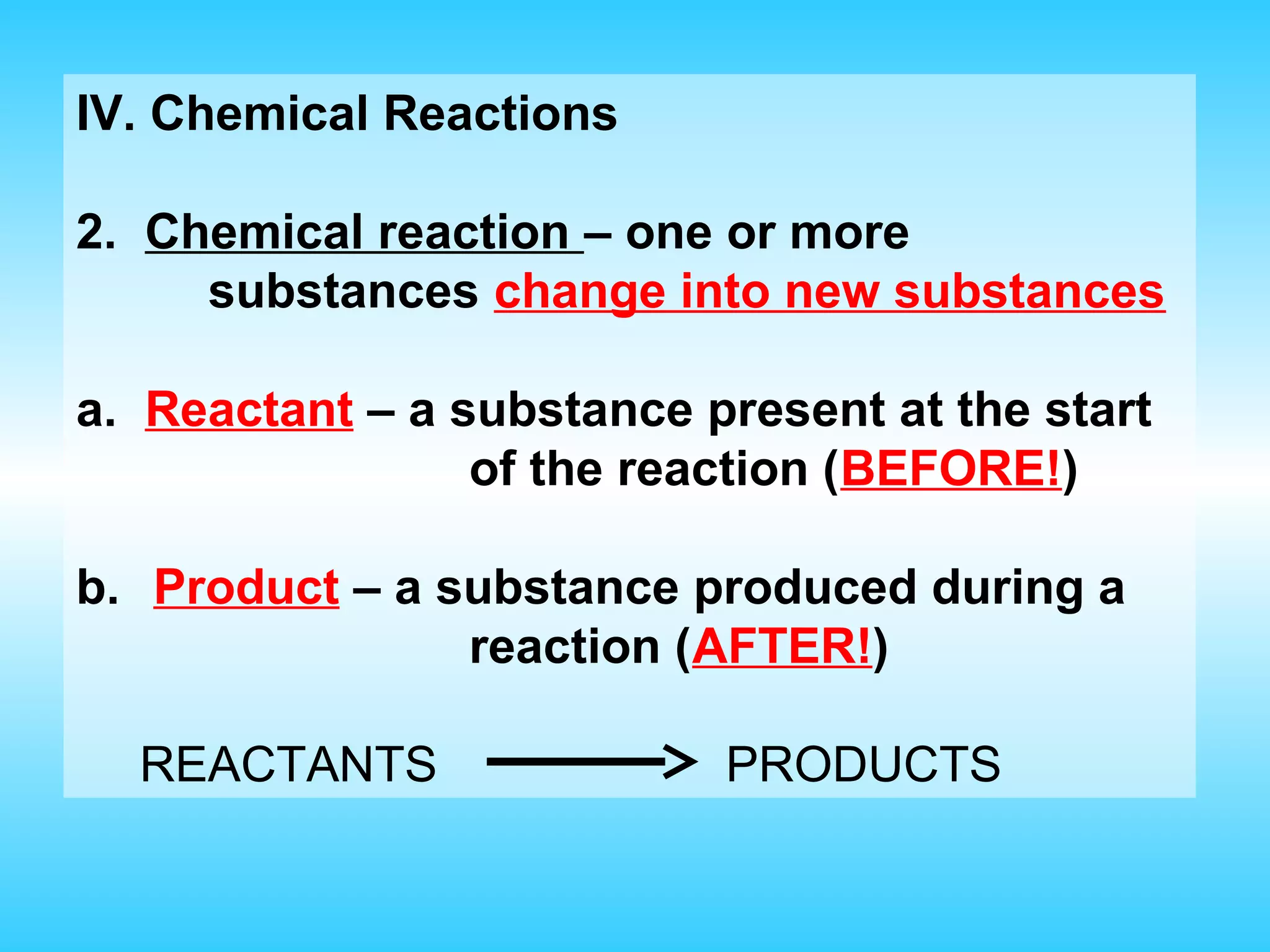
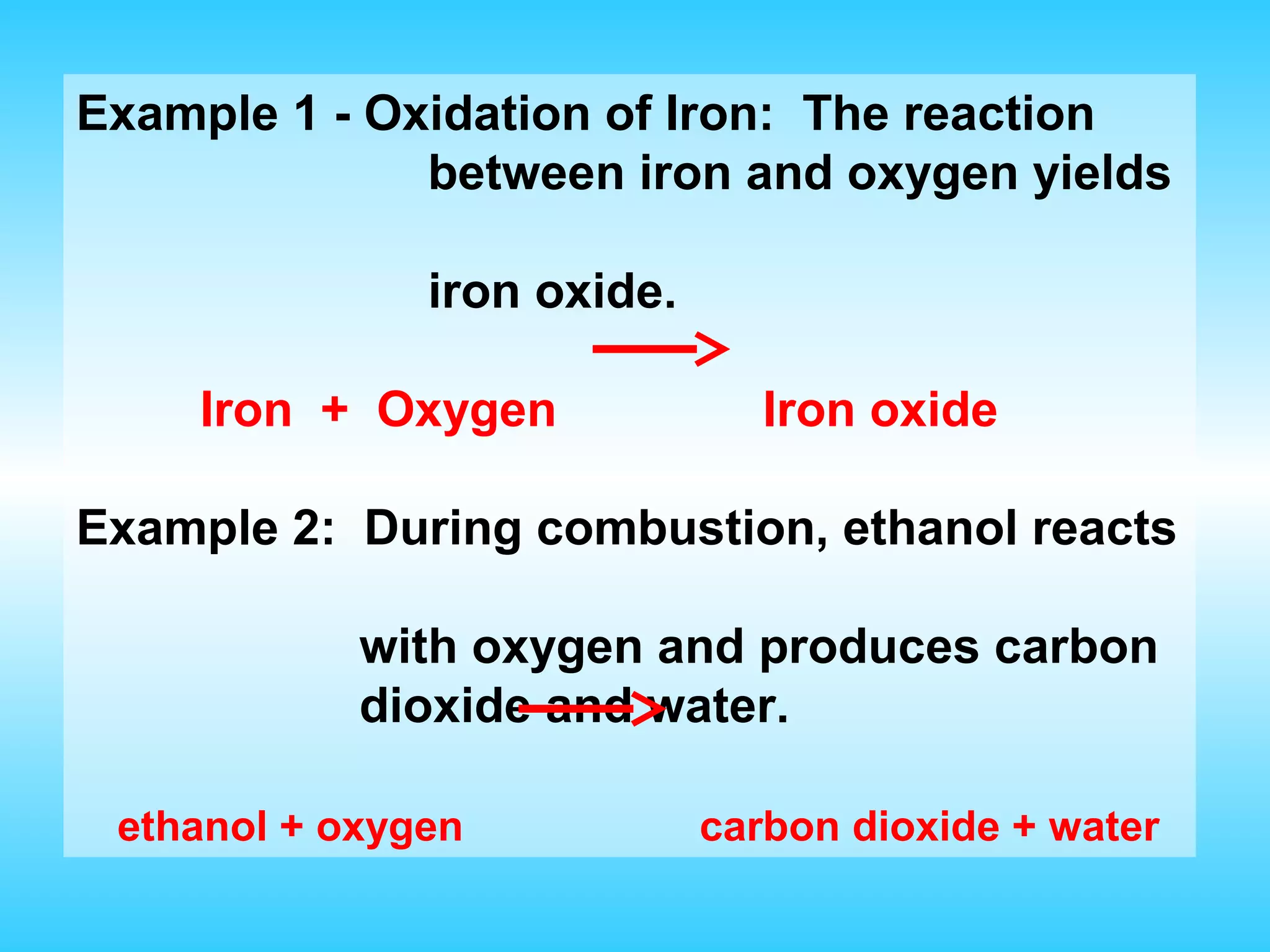
Chapter 2 discusses matter and its properties, defining matter as anything with mass and volume. It explains the classifications of substances into elements, compounds, and mixtures, highlighting their characteristics and methods of separation. The chapter also covers physical and chemical changes, chemical reactions, and the conservation of mass, illustrating key concepts with examples.