Embed presentation
Download to read offline


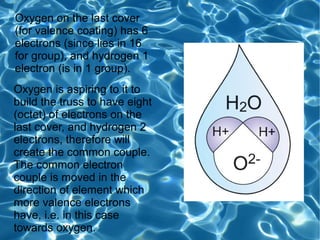



The water molecule is made of one oxygen atom and two hydrogen atoms. Chemists have found that the angle between the bonds is 105 degrees. The structural formula shows oxygen in the center with two hydrogen atoms bonded to it. A covalent bond is formed as the electrons are shared between the oxygen and hydrogen atoms due to their differing valences.





