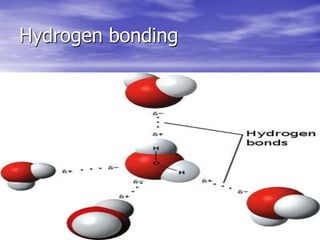
This document discusses the structure and properties of water molecules. It explains that water molecules are made up of an oxygen atom bonded to two hydrogen atoms, giving the oxygen a partial negative charge and the hydrogens a partial positive charge. This allows the molecules to form hydrogen bonds with neighboring water molecules. The hydrogen bonding between water molecules forms a three-dimensional network and gives water its unusual properties, such as its high heat capacity and boiling point. The dynamic hydrogen bonding allows water to easily flow and change states while maintaining its cohesive liquid properties.