The document discusses several interesting facts about water molecules and their structure:
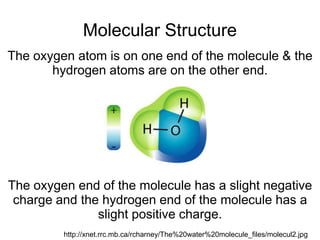
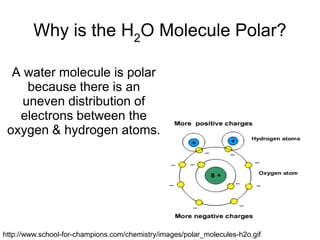
- Water molecules are polar, with hydrogen atoms having a slight positive charge and oxygen having a slight negative charge. This allows water molecules to form hydrogen bonds with other water molecules.
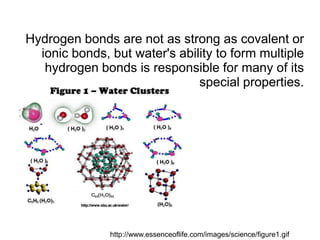
- A single water molecule can form up to four hydrogen bonds simultaneously. The ability of water to form these bonds gives it many unique properties.
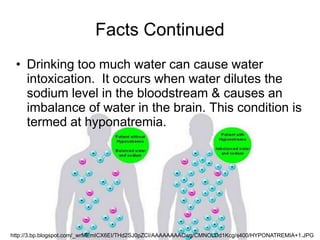
- Too much water consumption can cause a sodium imbalance in the blood known as hyponatremia. Most of the human body and planet are made up of water.