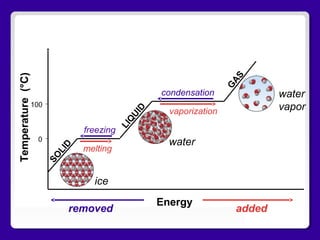
The document discusses the hydrosphere, which includes all of Earth's water found in oceans, glaciers, streams, lakes, soil, groundwater, living organisms, and air. It then describes the structure of water molecules and explains the phases of water (ice, liquid water, water vapor) and the physical changes (melting, freezing, vaporization, condensation) between phases that require energy input or output from the environment. Finally, it shows an energy-temperature graph illustrating the relationship between energy and state changes of water.