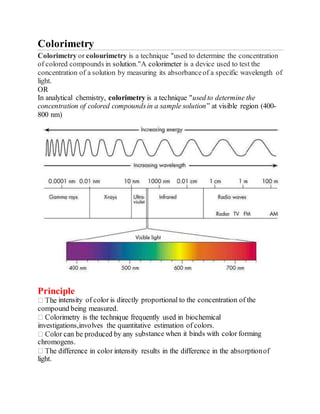
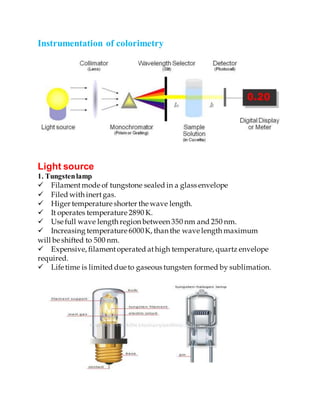
Colorimetry is a technique used to determine the concentration of colored compounds in solution by measuring the absorbance of light at specific wavelengths. The intensity of the color produced is directly proportional to the concentration of the compound being measured. Modern colorimetry uses a photoelectric cell to measure light absorption, replacing the human eye, and provides a direct measure of light intensity. Colorimetry is used in analytical chemistry applications such as determining concentrations of compounds in clinical samples and in industrial quality control.