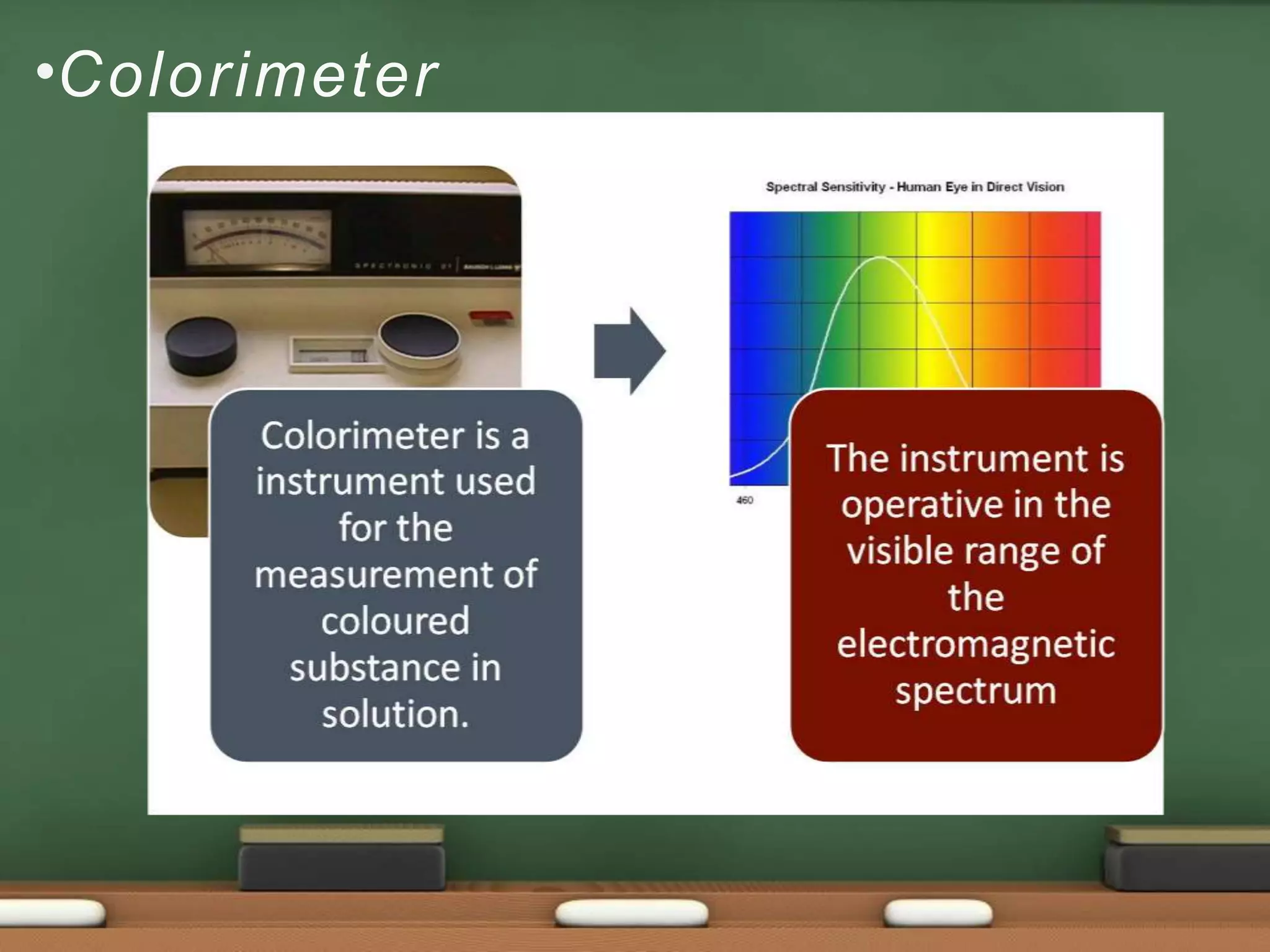
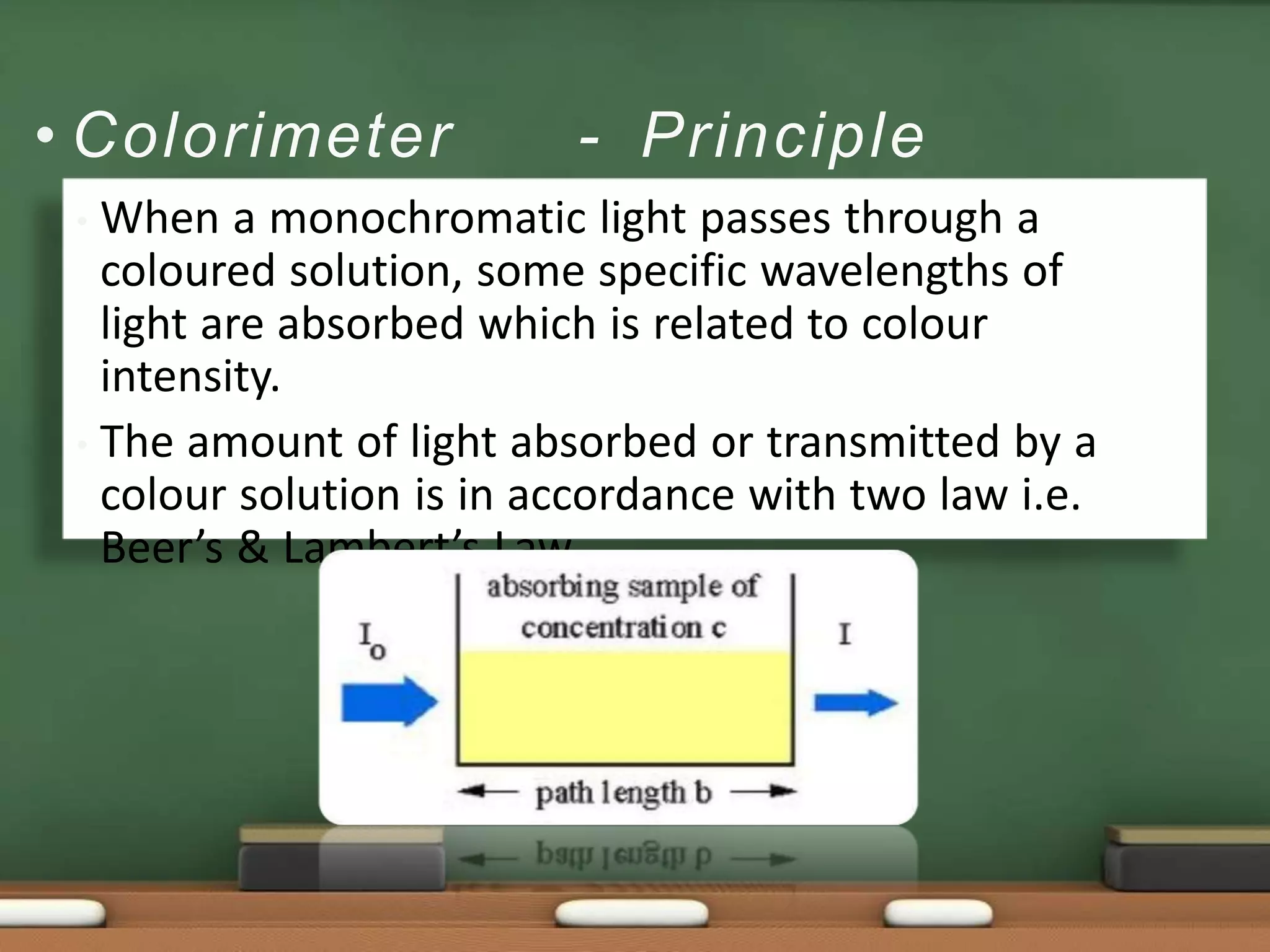
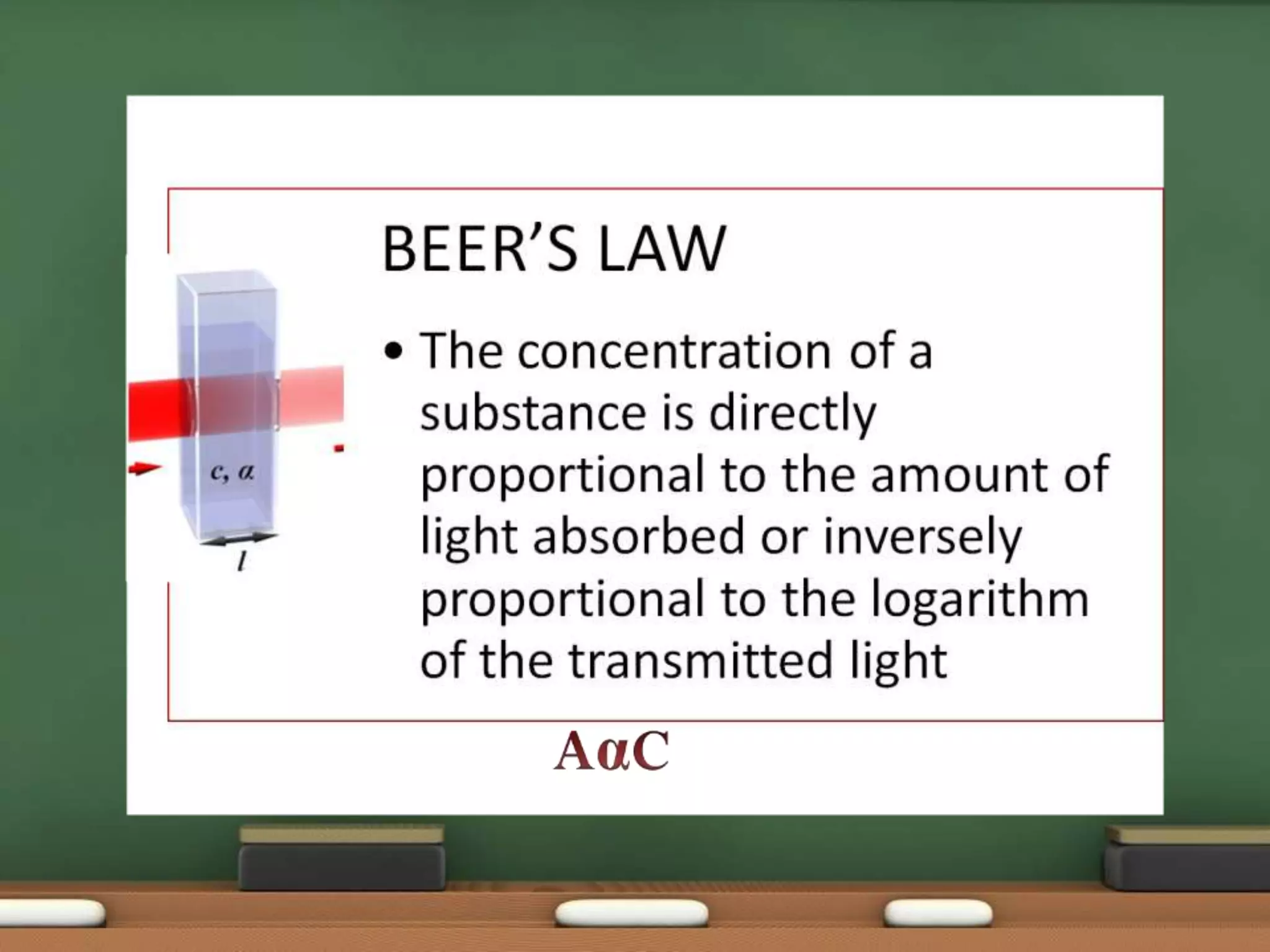
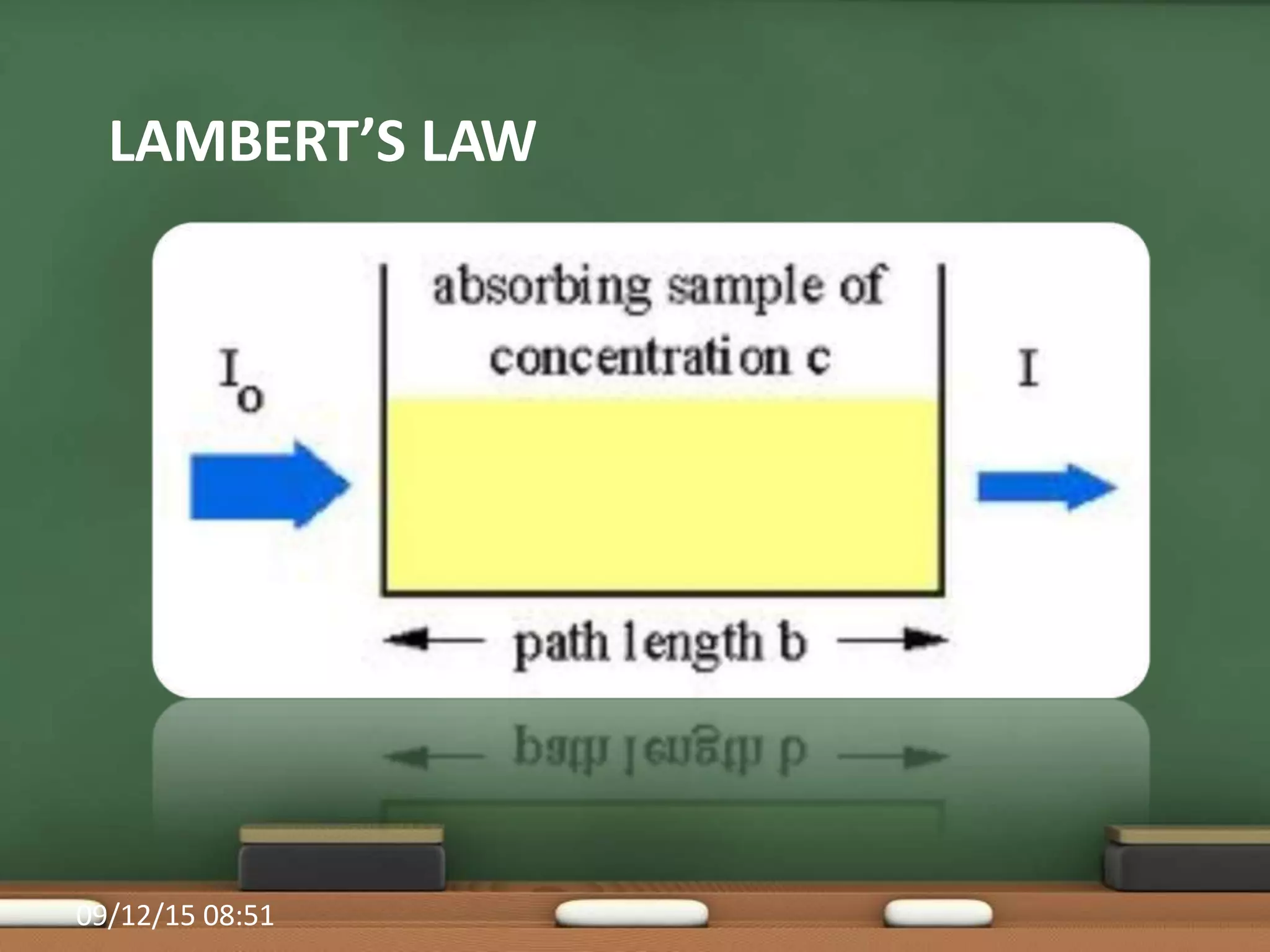
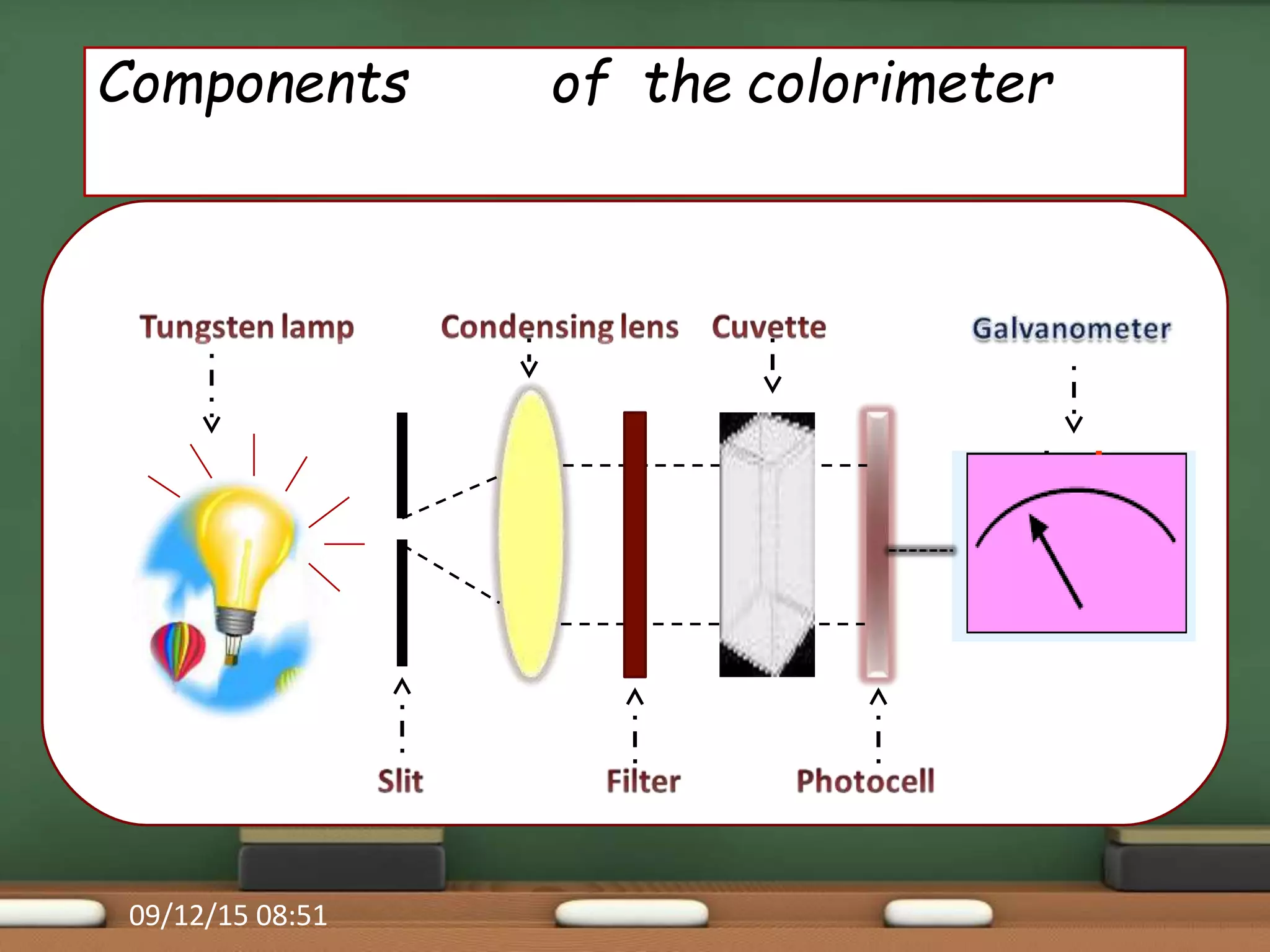
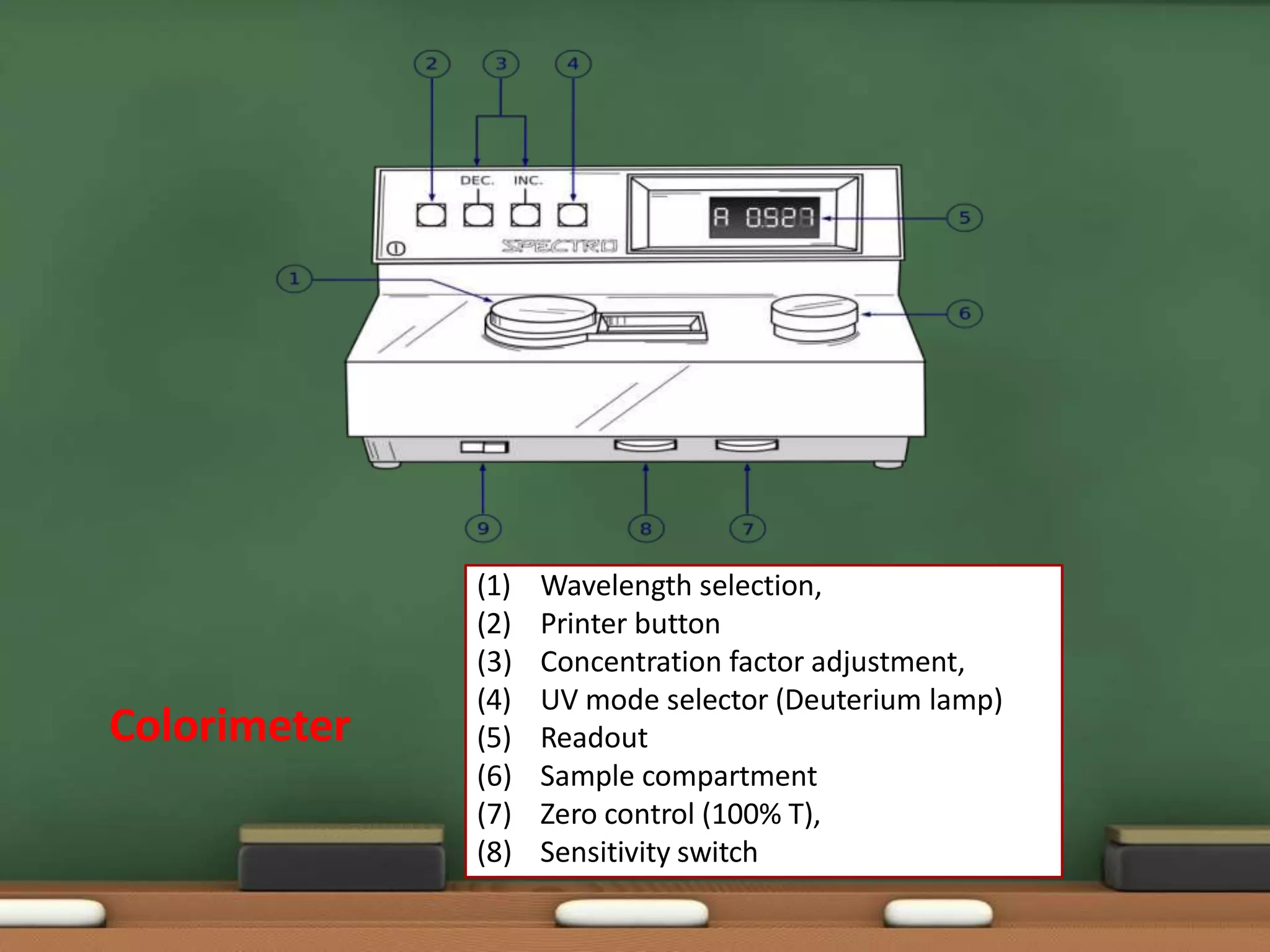
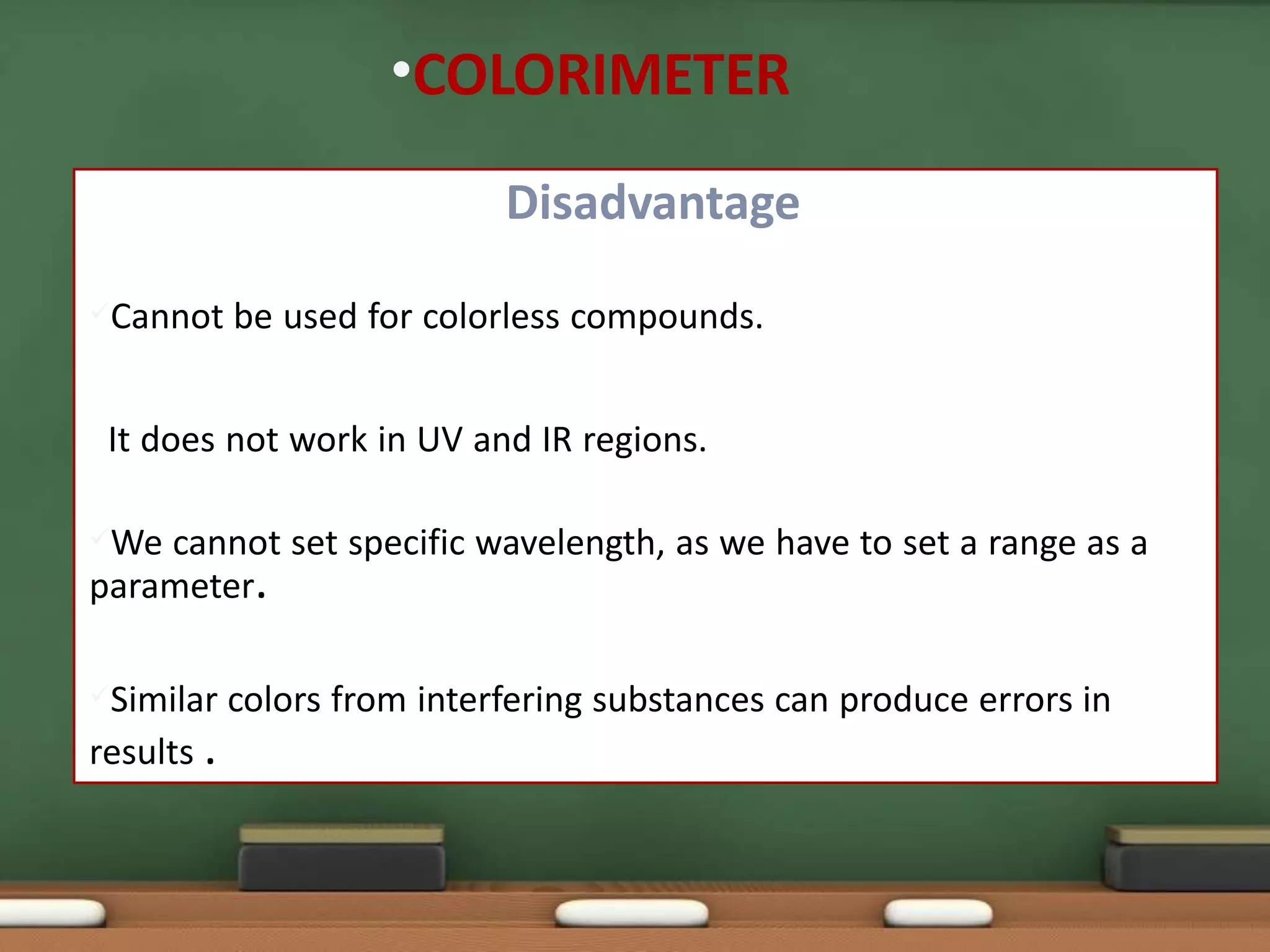
Colorimetry is a common analytical technique used in clinical laboratories to estimate the concentration of colored substances. It works by measuring how much light a colored solution absorbs, which follows Beer's and Lambert's laws - the amount of light absorbed increases with increasing concentration and path length of the solution. A colorimeter uses a light source, filters to select the wavelength, a sample holder cuvette, and detector to measure the light absorbed and calculate the concentration. Regular maintenance such as cleaning optics and replacing worn parts is needed to ensure accurate operation.