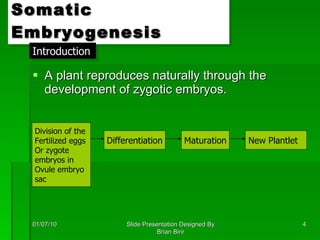
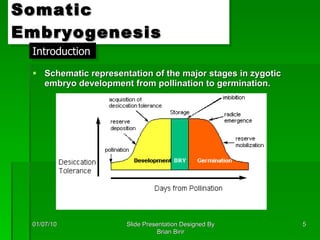
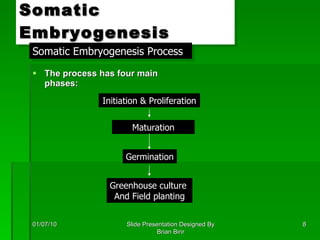
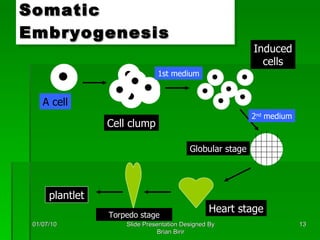
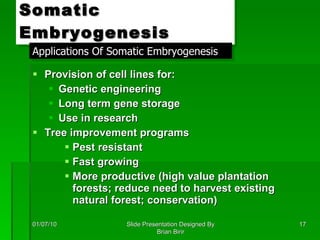
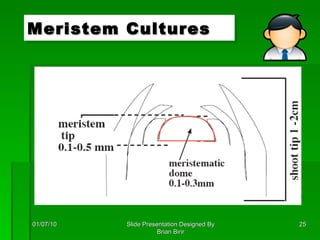
The document discusses clonal propagation techniques, particularly somatic embryogenesis and meristem cultures, which allow for the production of genetically identical plants from a single somatic cell or meristem. It outlines the phases of somatic embryogenesis, including initiation, maturation, and germination, and explains the significance of these methods in reducing genetic variability and accelerating plant production. Additionally, it highlights the applications of these techniques in genetic engineering and improving plant traits such as pest resistance and growth efficiency.