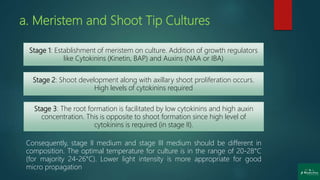
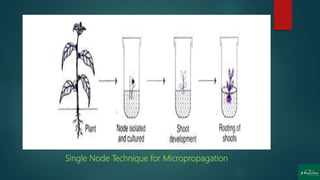
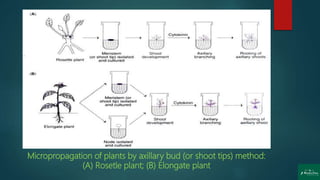
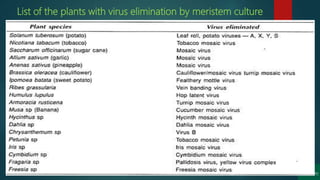
The document discusses clonal propagation techniques, particularly focusing on micropropagation through tissue culture as a method for rapid plant multiplication. It details the advantages of clonal propagation, including the creation of genetically identical plants and the avoidance of juvenile phases, and outlines various methods of achieving this, such as organogenesis and somatic embryogenesis. Furthermore, it addresses the stages involved in micropropagation, from explant preparation to transplantation, and highlights the commercial significance of this technique in the horticultural industry.




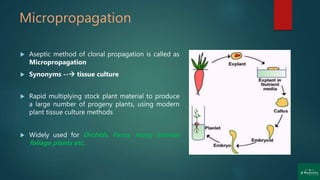






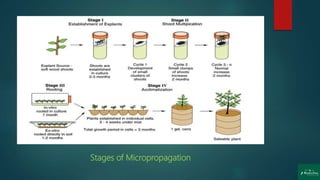
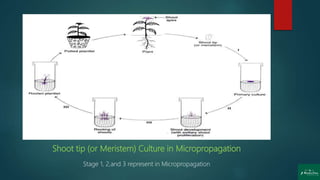
![Stages of Micropropagation
Stage 0: Preparation of the mother plant
Stage 1: Initiation and establishment of cultures
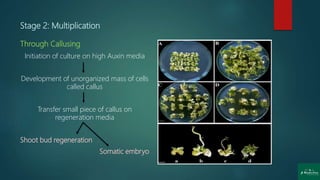
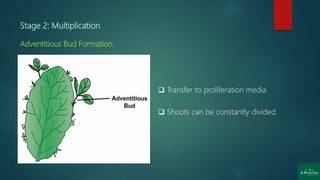
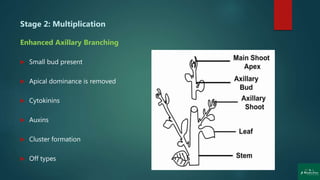
Stage 2: Multiplication [shoot or rapid somatic embryo formation]
Stage 3: In-vitro germination of somatic embryo
and rooting of shoots
Stage 4: Transfer of plantlets to sterilized soil for Hardening
under greenhouse/ field conditions (transplantation)](https://image.slidesharecdn.com/micropropagation-200904052317/85/Clonal-Propagation-Introduction-Techniques-Factors-Applications-and-Disadvantages-13-320.jpg)