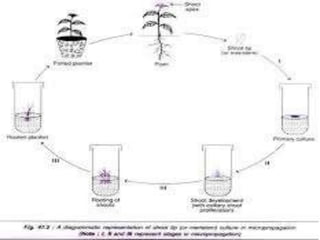
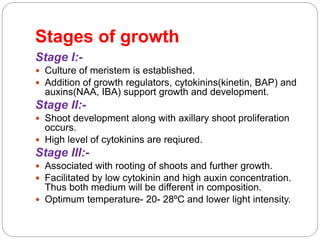
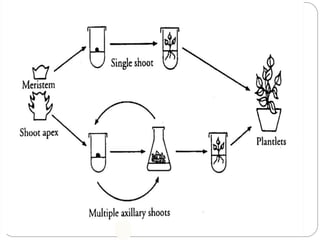
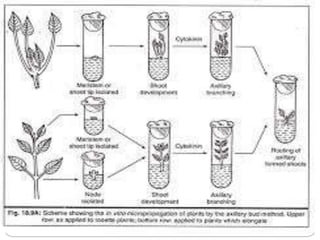
This document discusses micropropagation, which is the rapid multiplication of plant materials using tissue culture methods. It involves taking explants like shoot tips or buds and culturing them on growth media to produce many new plantlets. The process involves initiation, multiplication, rooting, and acclimatization stages. Approaches include multiplication from axillary buds/shoots or adventitious shoots. Applications are high rate propagation of disease-free plants, seed production in some crops, and cost effectiveness. Automation using bioreactors and robots can increase production scale but reduces flexibility.