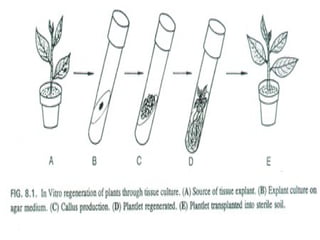
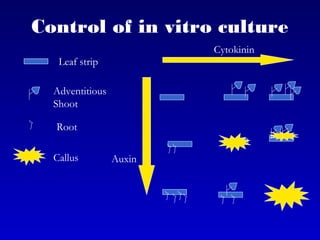
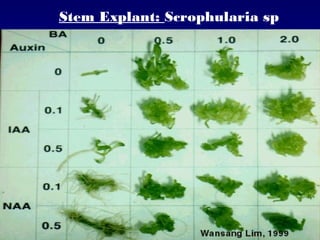
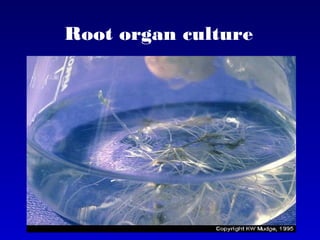
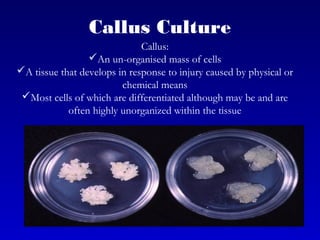
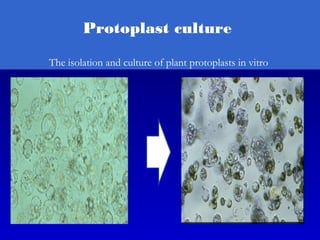
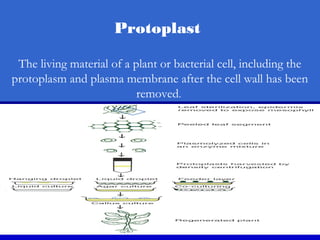
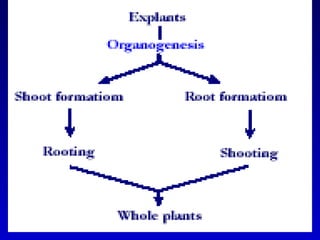
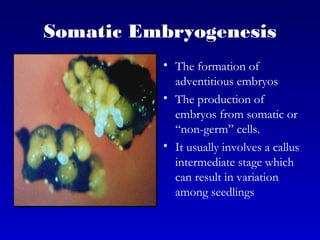
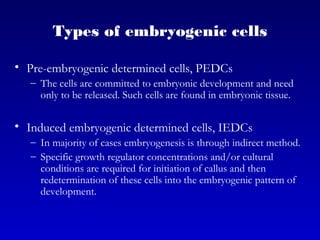
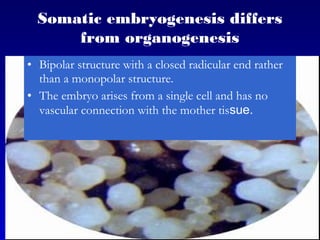
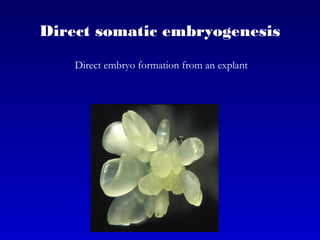
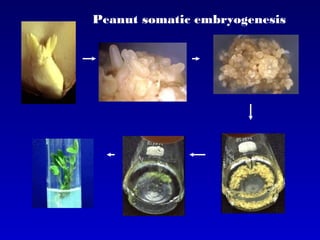
This document discusses in vitro plant breeding techniques. It describes in vitro culture as the growth and development of plant tissues, cells, or protoplasts under controlled artificial conditions using glass or plastic vessels. The key abilities of plants that allow for in vitro culture are totipotency, dedifferentiation, and competency. Important factors for successful in vitro culture include growth media, environmental conditions, and the explant source. The document outlines various types of in vitro culture including seed, embryo, organ, callus, cell suspension, and protoplast cultures. It also describes plant regeneration pathways such as microcutting, organogenesis, and somatic embryogenesis.