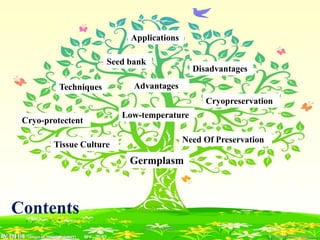
1) Germplasm conservation involves preserving genetic material, such as seeds, cells, tissues, and body parts, through in-situ and ex-situ methods to maintain biodiversity and provide resources for breeding programs.
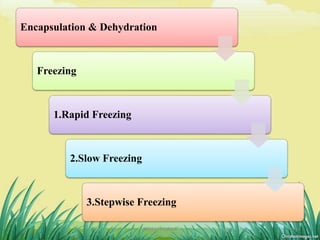
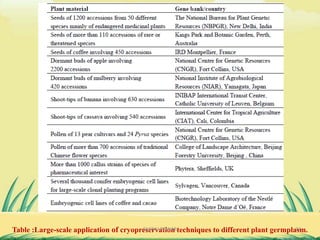
2) Cryopreservation at ultra-low temperatures in liquid nitrogen is an important ex-situ technique that can preserve germplasm long-term without subculturing. It involves preculturing plant materials, treating with cryoprotectants, and either slow-freezing or vitrification prior to storage in liquid nitrogen.
3) A case study demonstrates the successful cryopreservation of mint shoot tips using encapsulation-dehydration and PVS2-vitrification, with