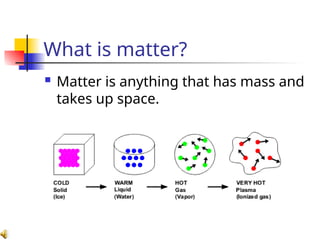
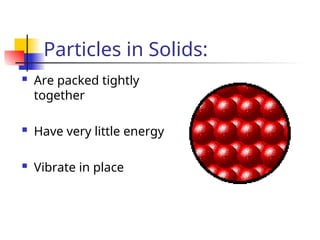
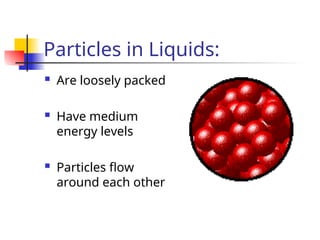
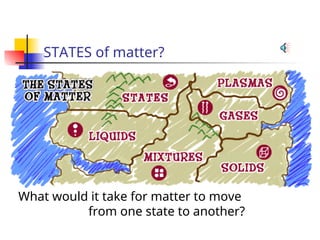
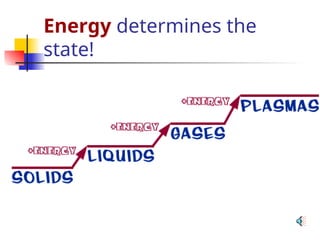
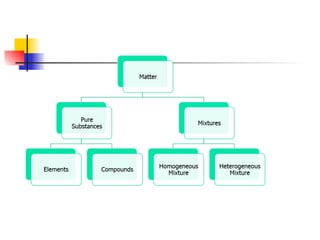
The document provides an overview of matter and its properties, detailing branches of chemistry like organic, inorganic, and analytical chemistry. It describes the four states of matter—solids, liquids, gases, and plasma—along with their characteristics and the physical and chemical properties used for identification. Additionally, it discusses the changes between states of matter, the classification of substances, and the differences between pure substances, compounds, and mixtures.