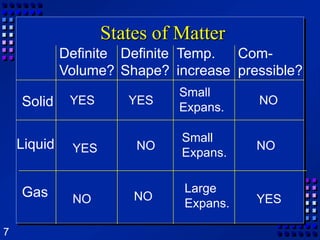
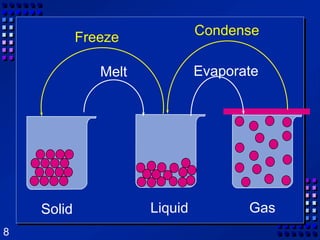
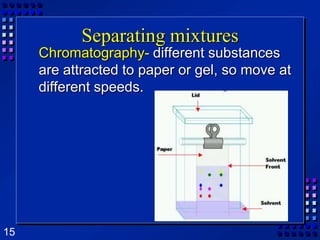
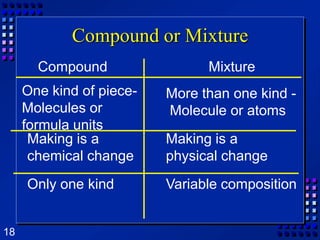
This document provides an overview of matter and changes to matter. It defines key concepts such as elements, compounds, mixtures, physical and chemical properties, and states of matter. It also describes different types of physical and chemical changes, including phase changes, and explains techniques for separating mixtures like filtration, distillation, and crystallization. The document also introduces the concepts of energy and its various forms, and explains the laws of conservation of mass and energy.