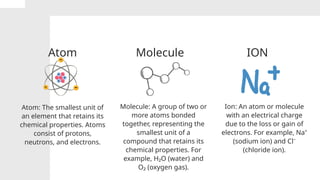
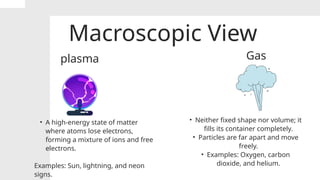
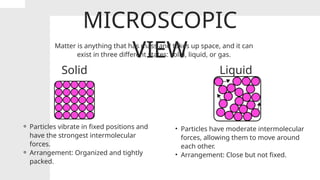
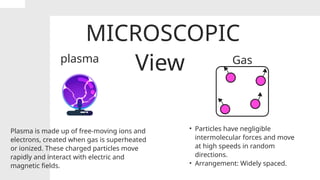
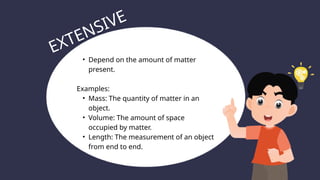
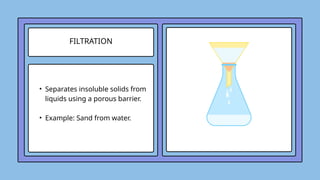
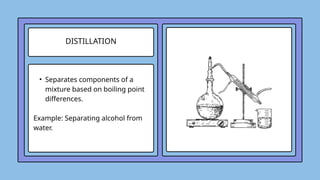
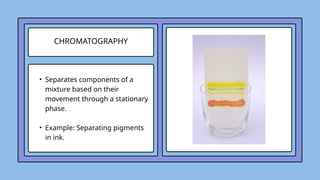
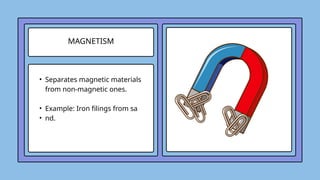
The document covers the particulate nature of matter, including its properties, states (solid, liquid, gas, plasma), and methods of separating mixtures. It distinguishes between physical and chemical properties, as well as pure substances and mixtures, detailing examples of each. Additionally, it explains physical separation techniques such as filtration, distillation, and chromatography.