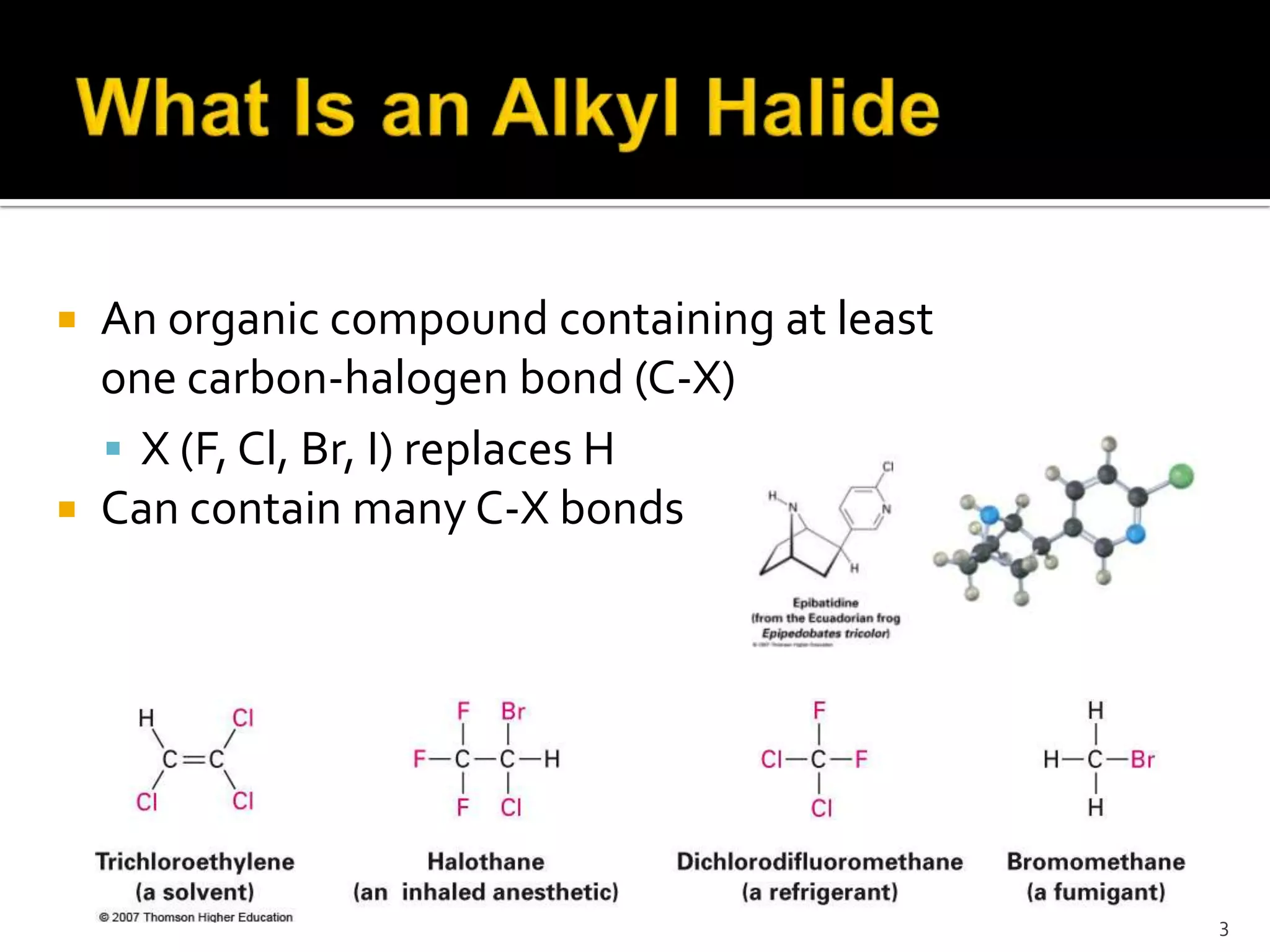
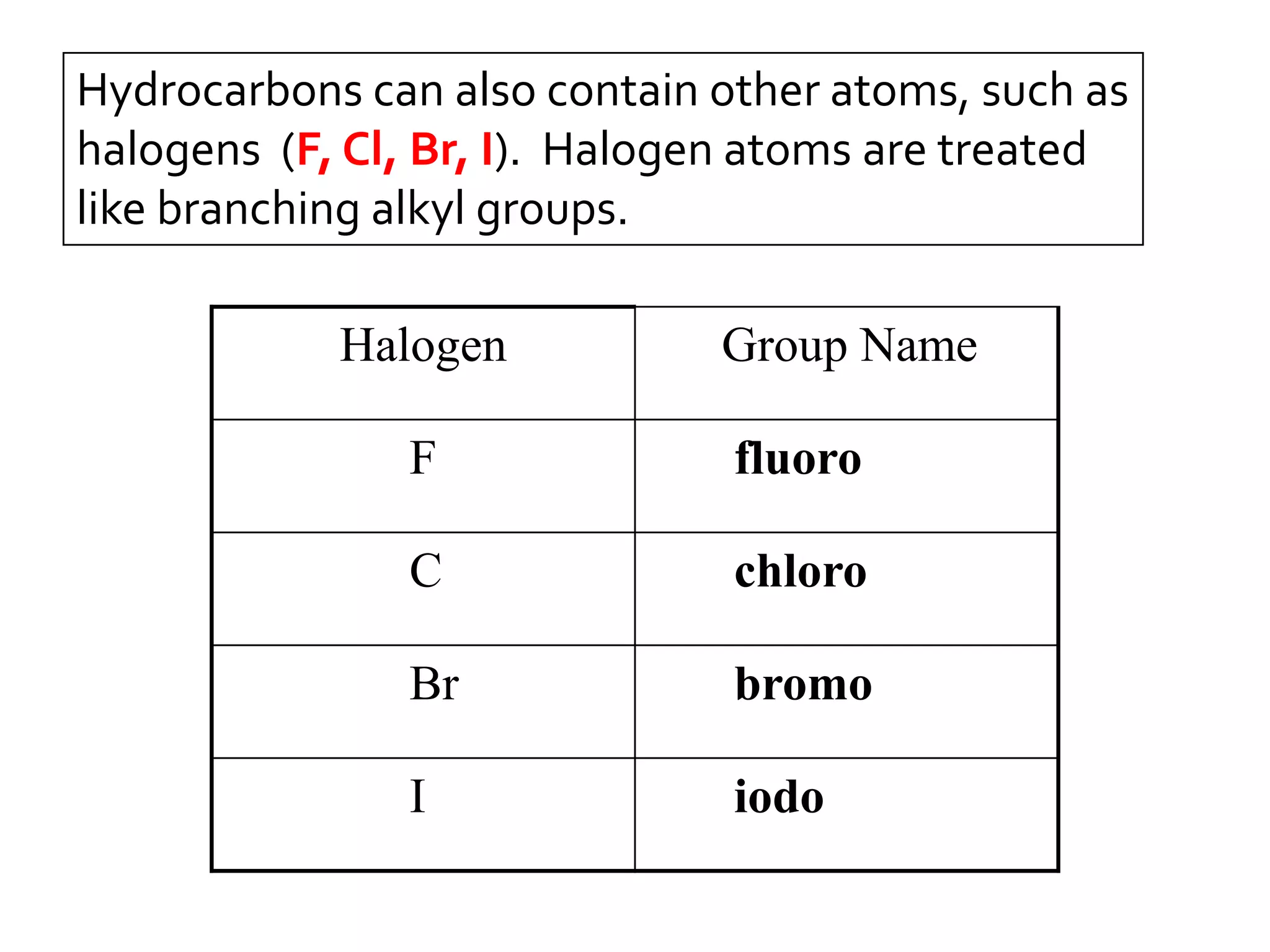
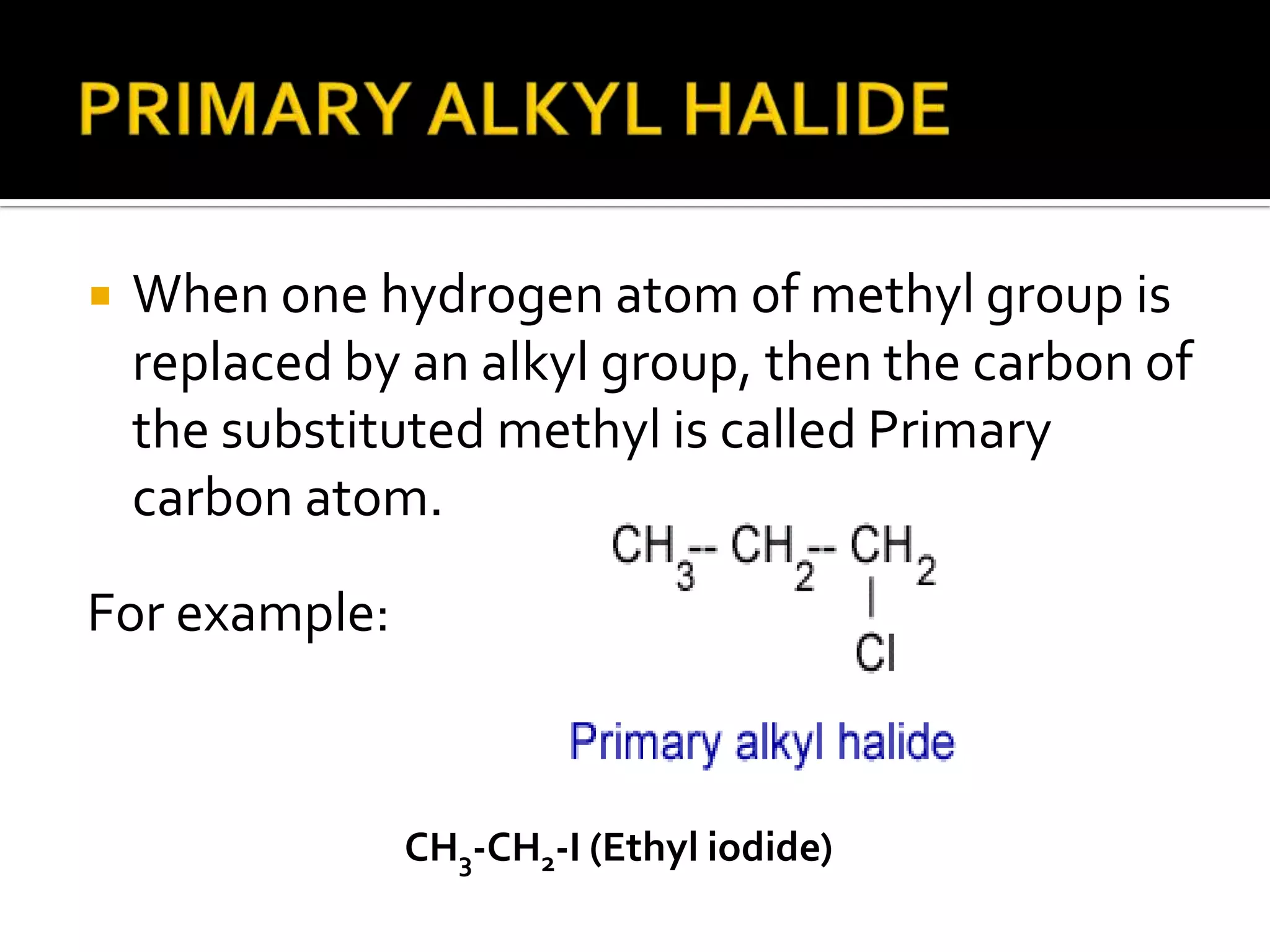
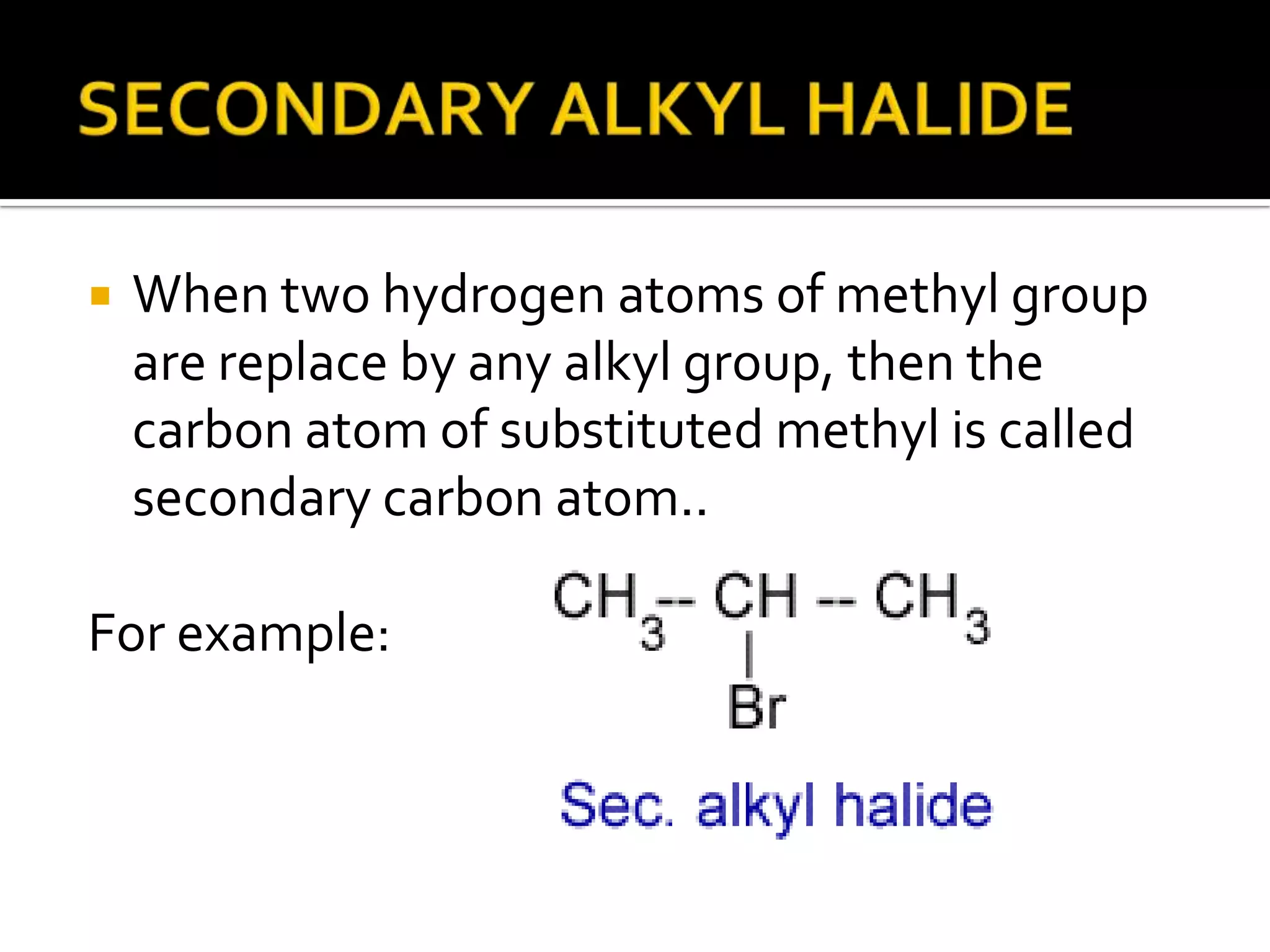
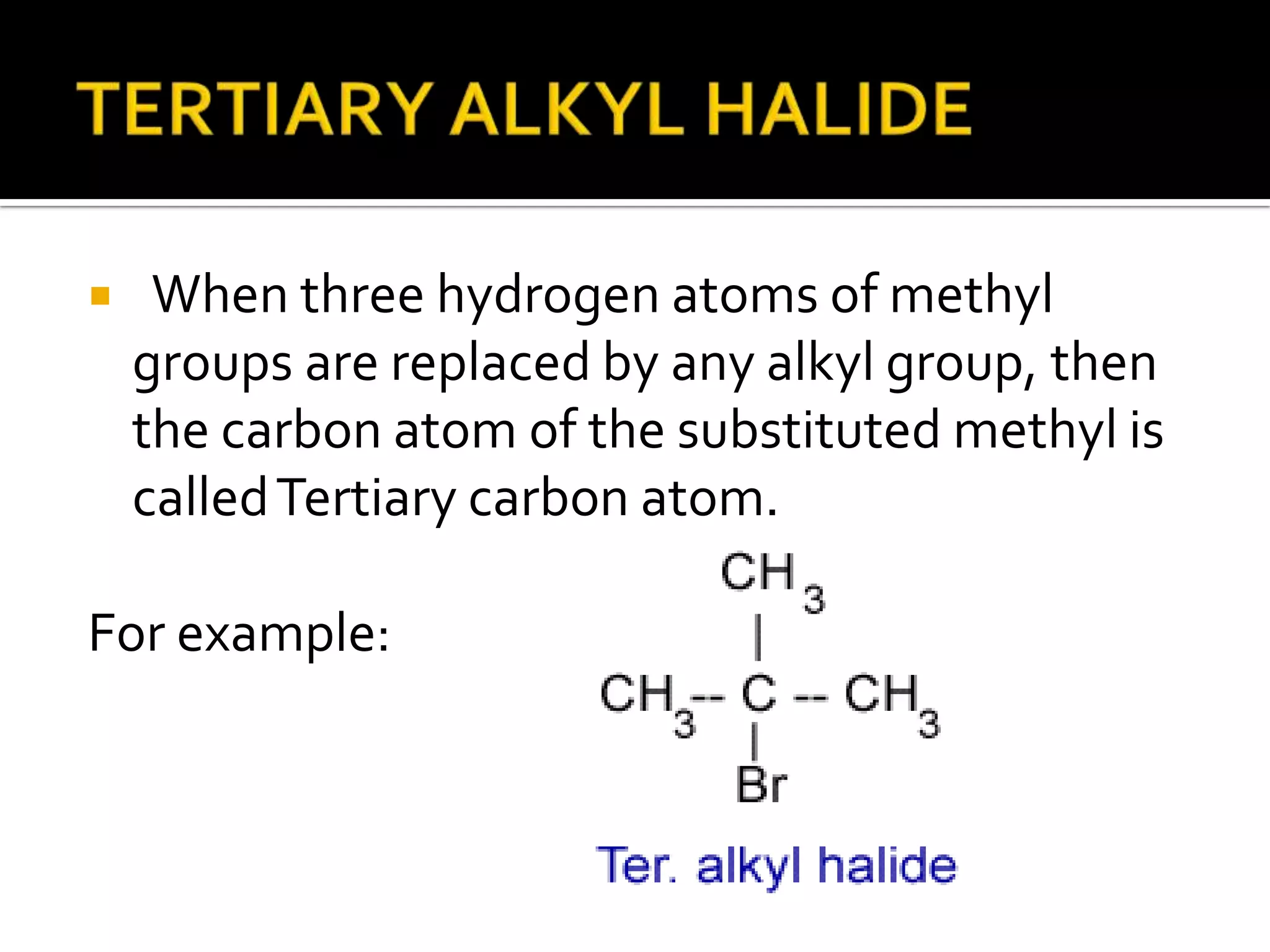
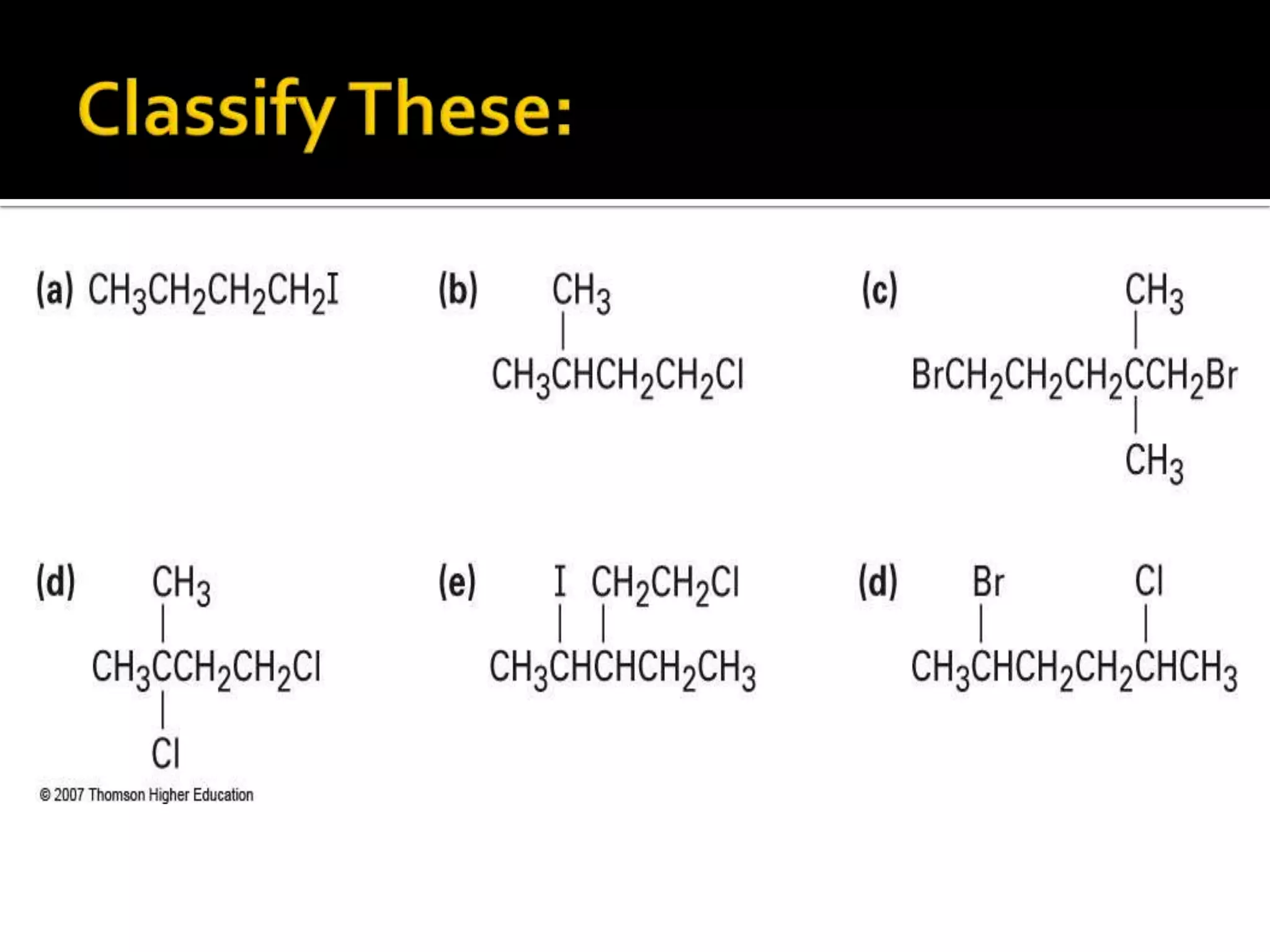
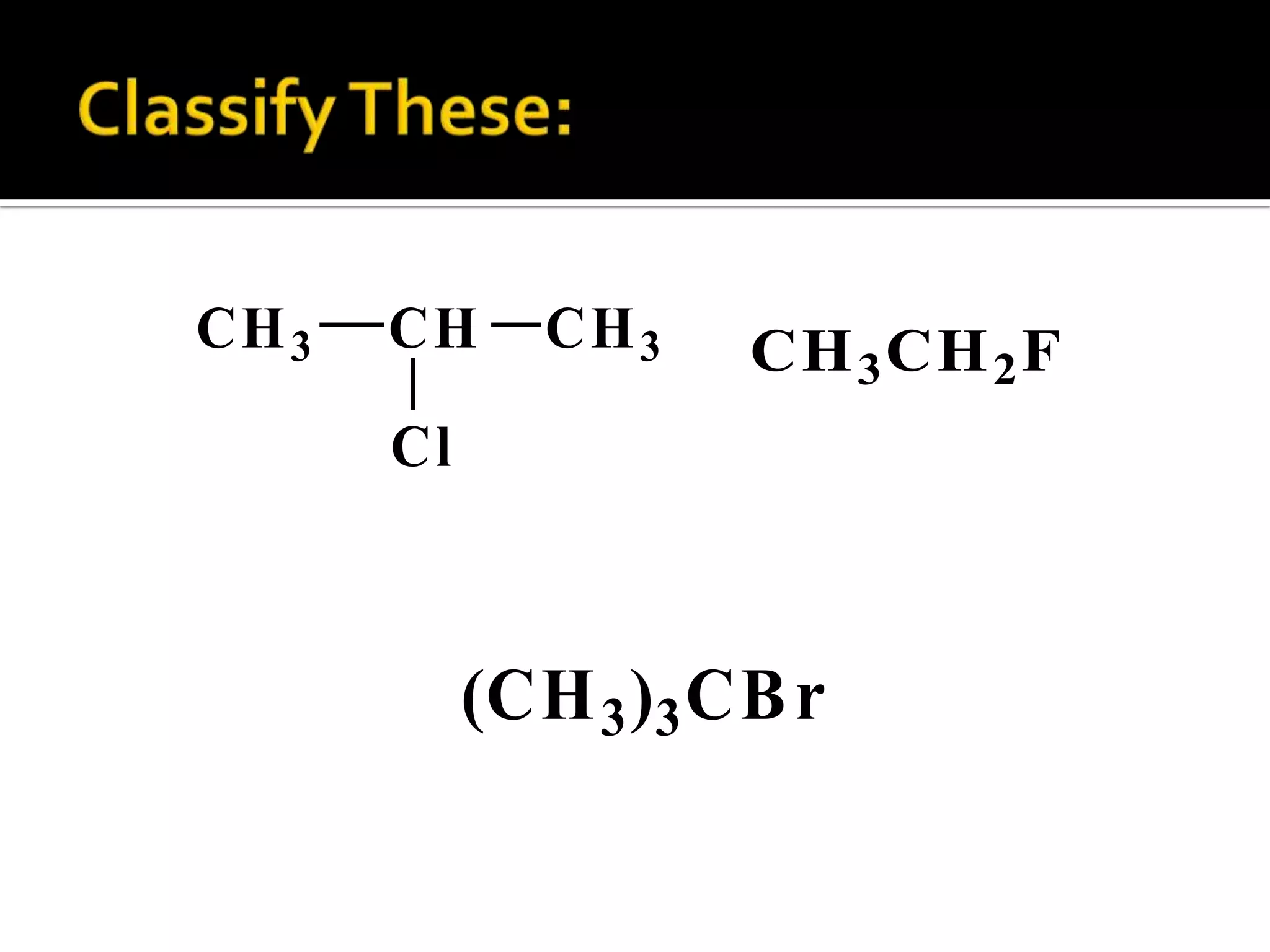
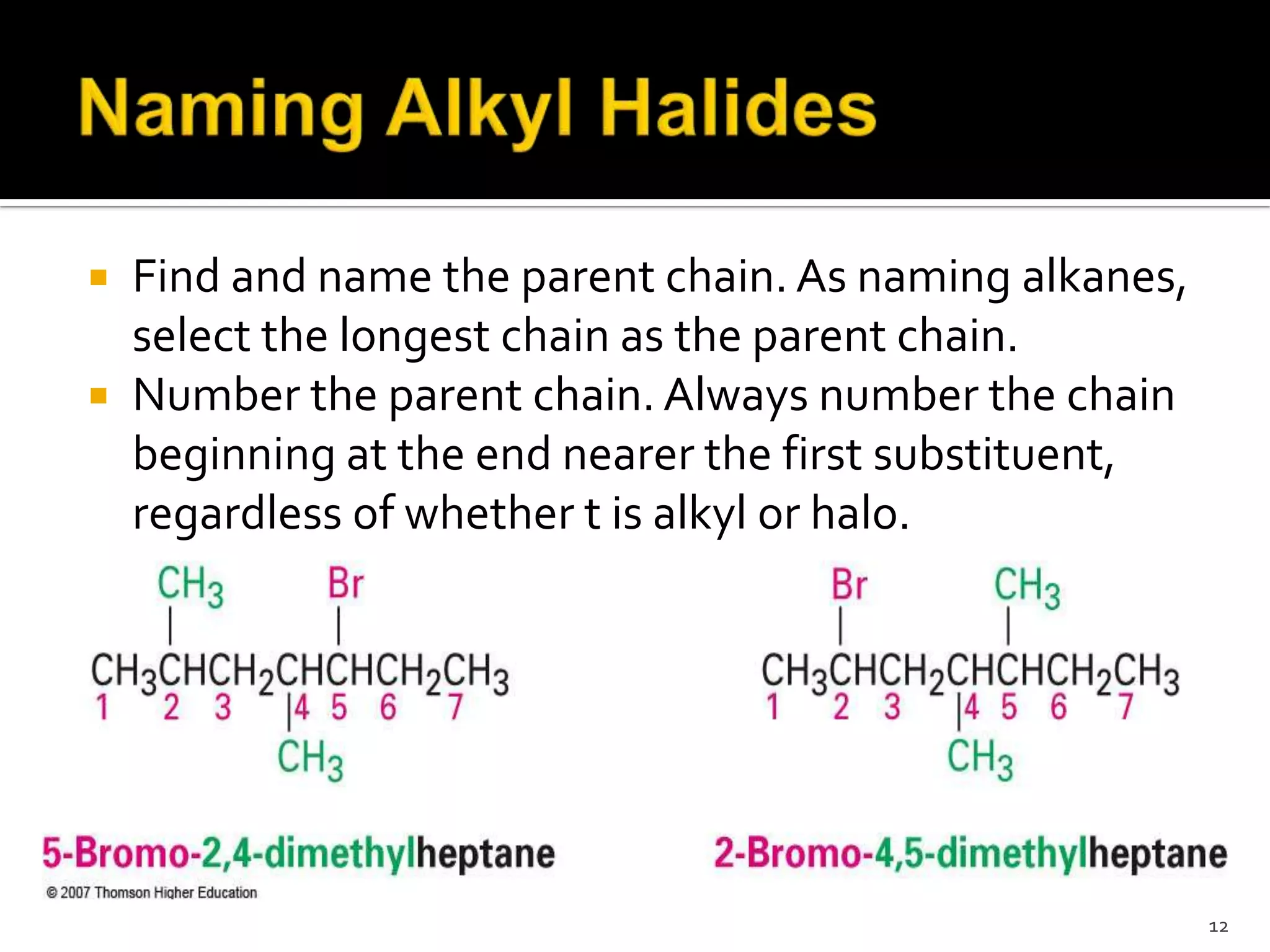
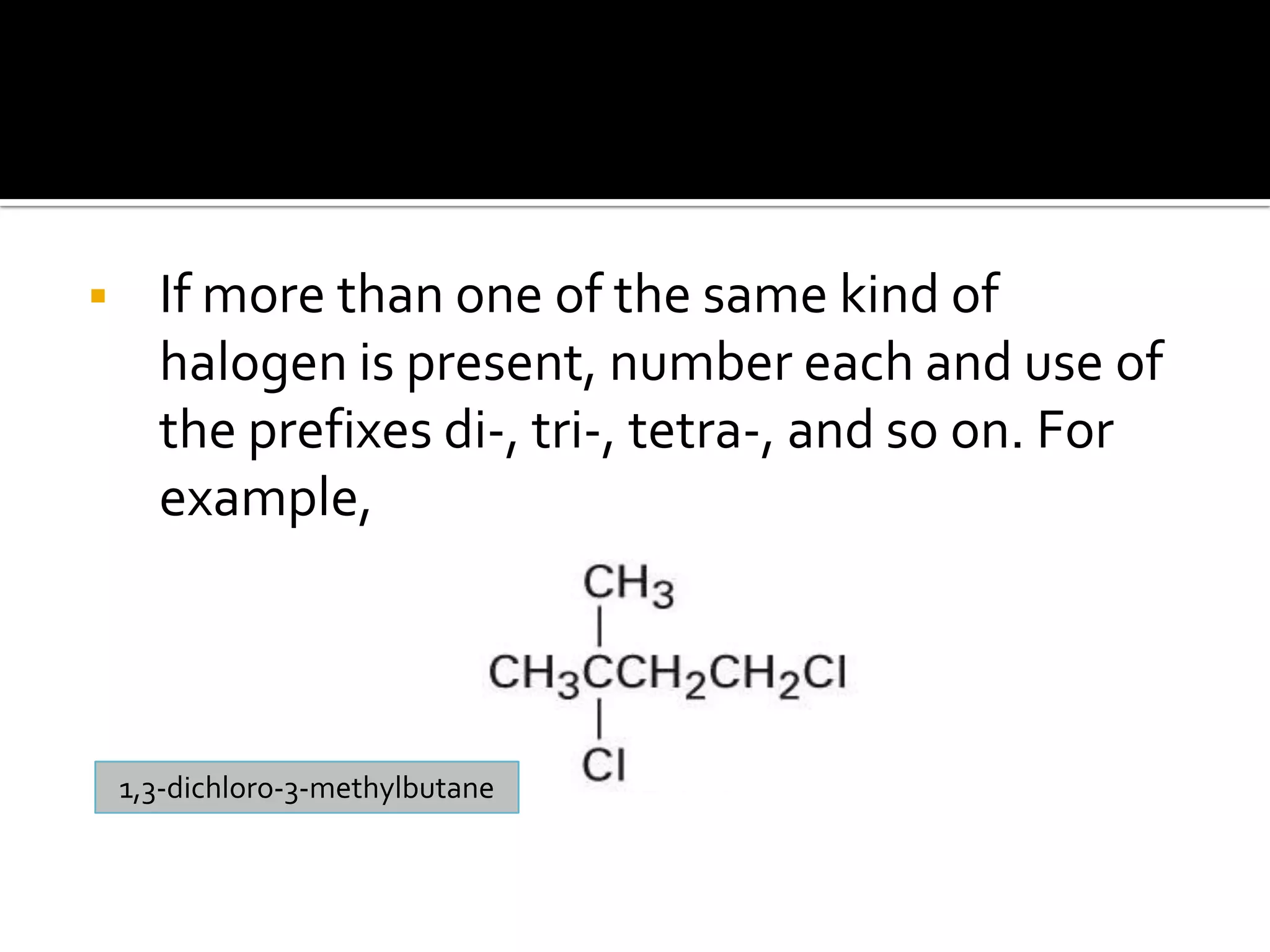
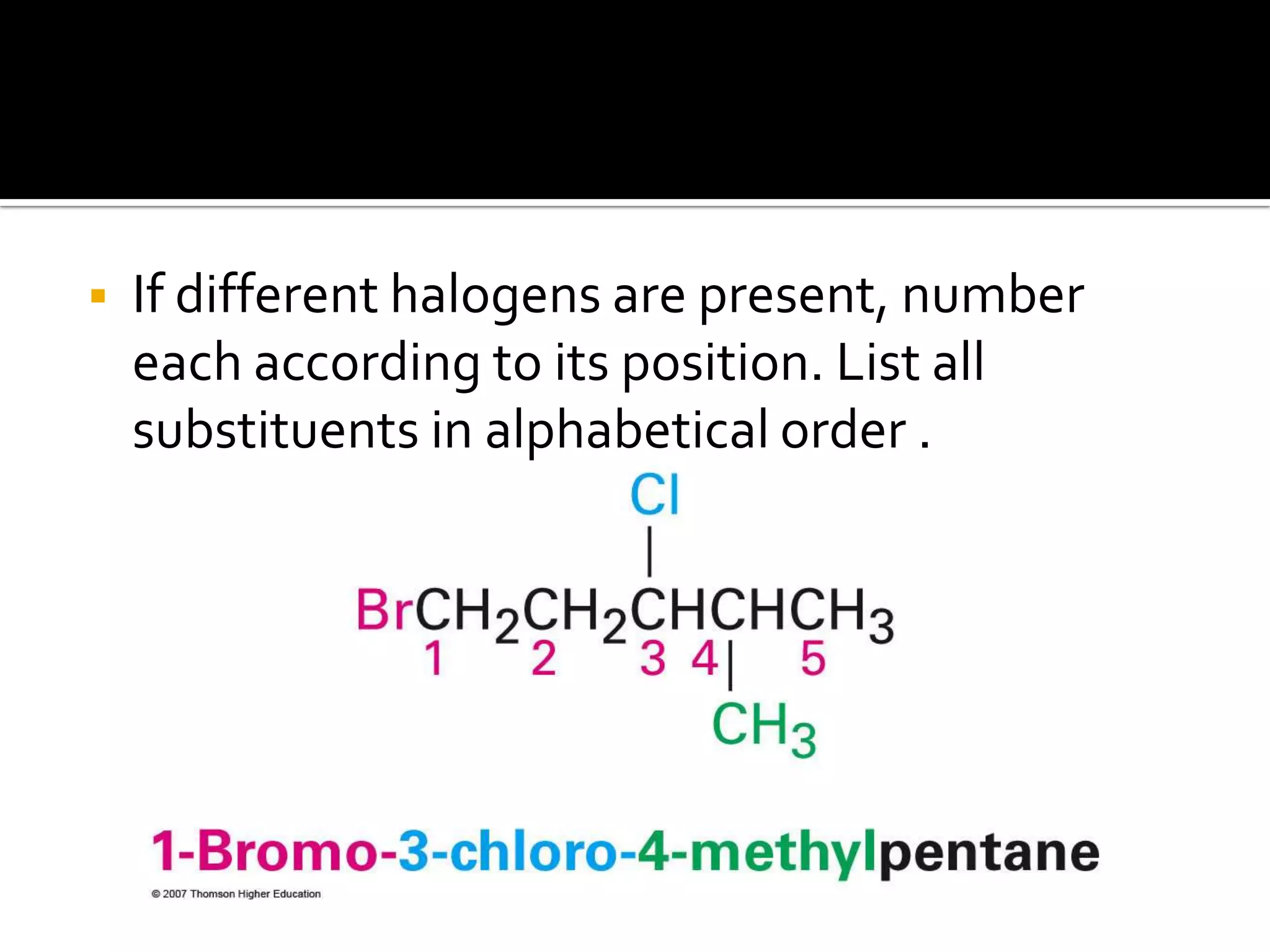
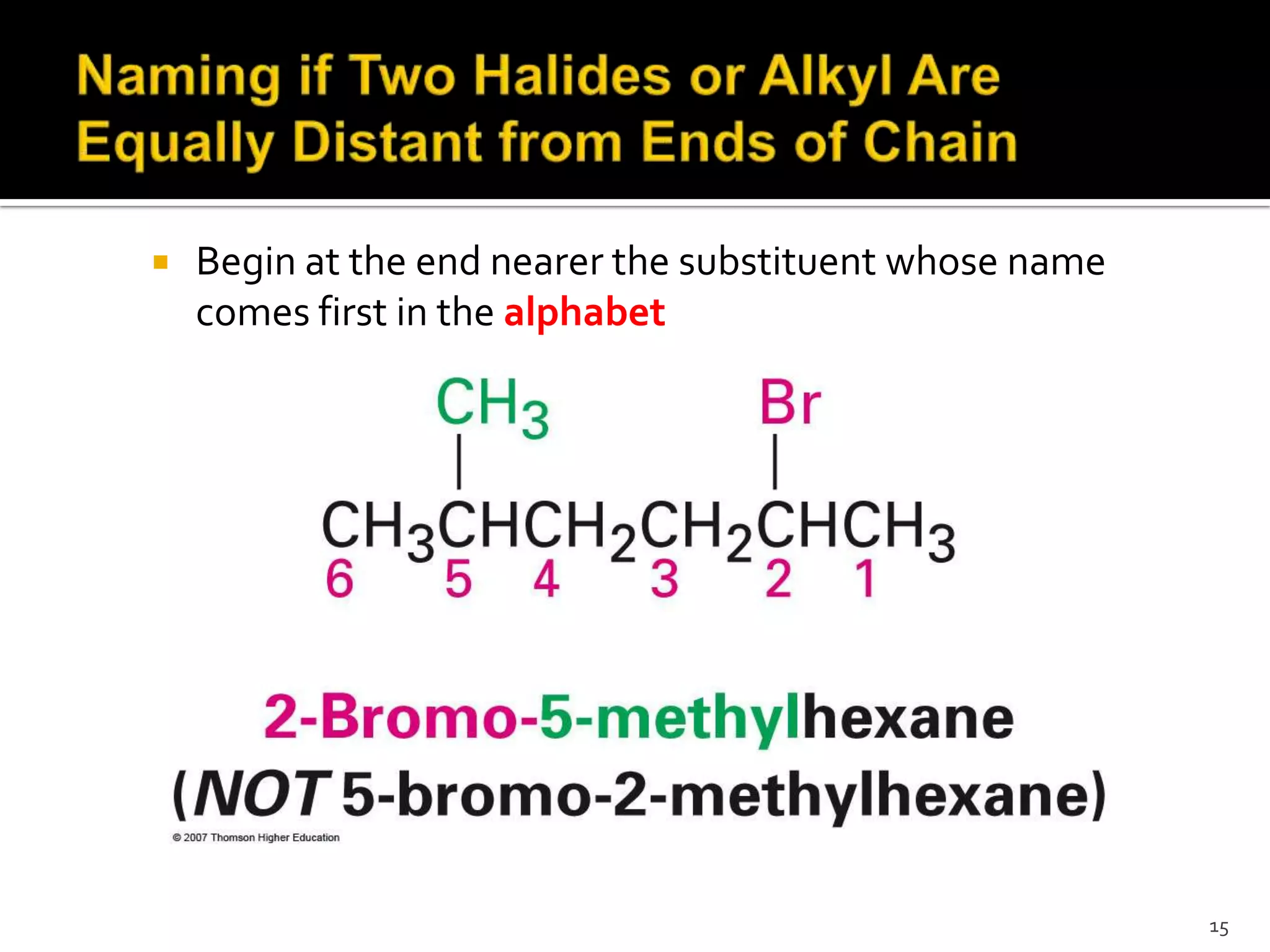
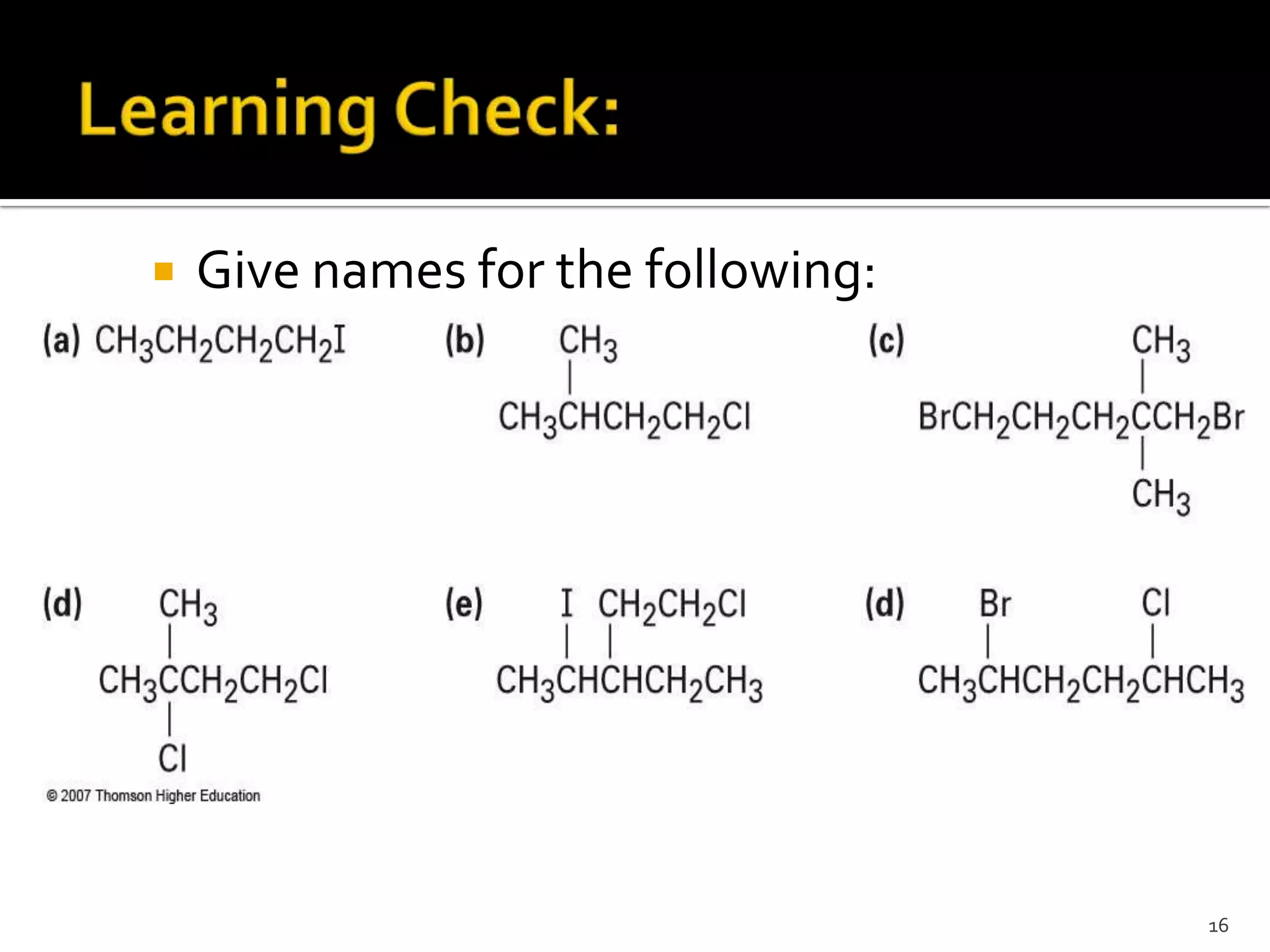
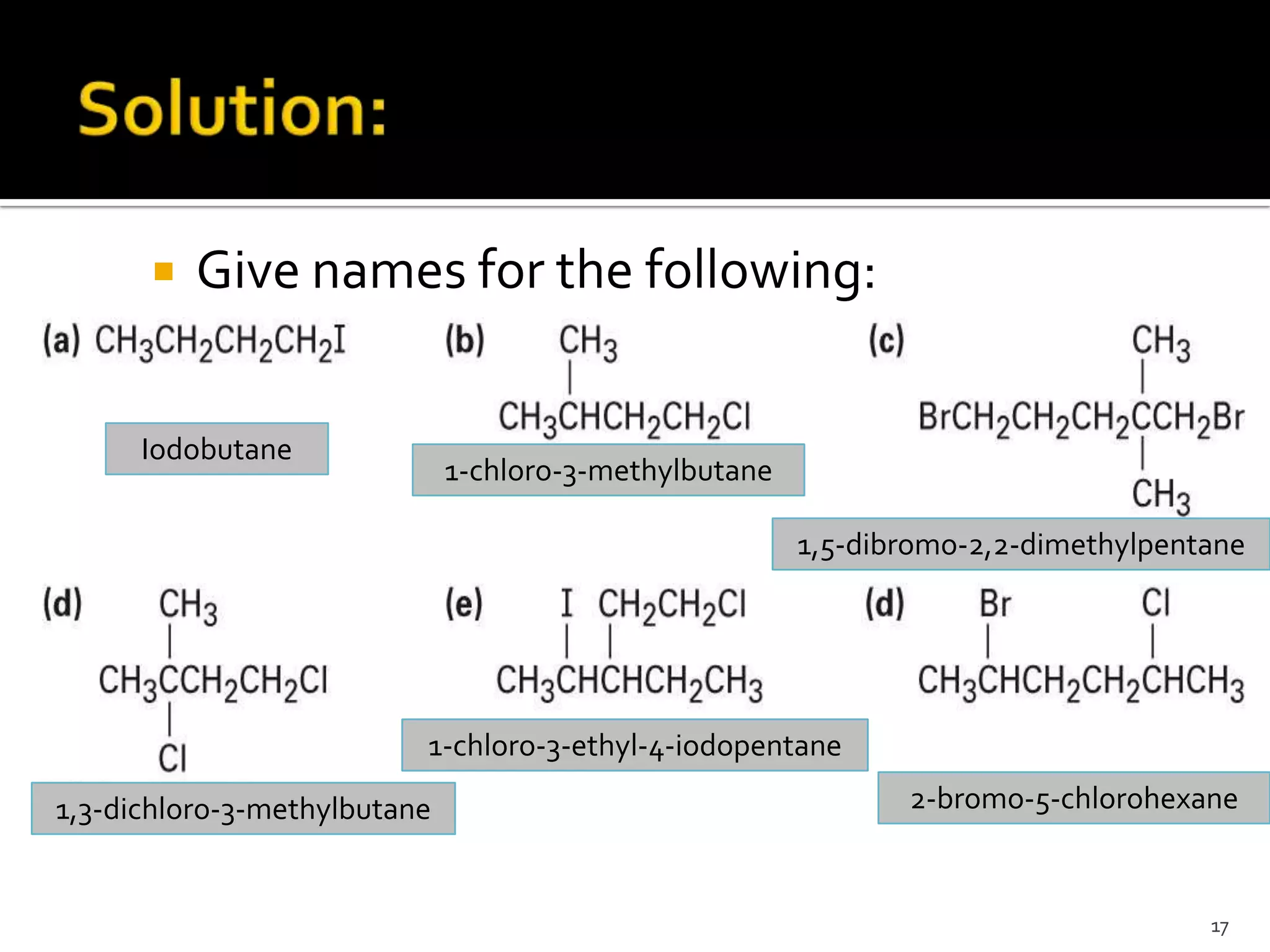
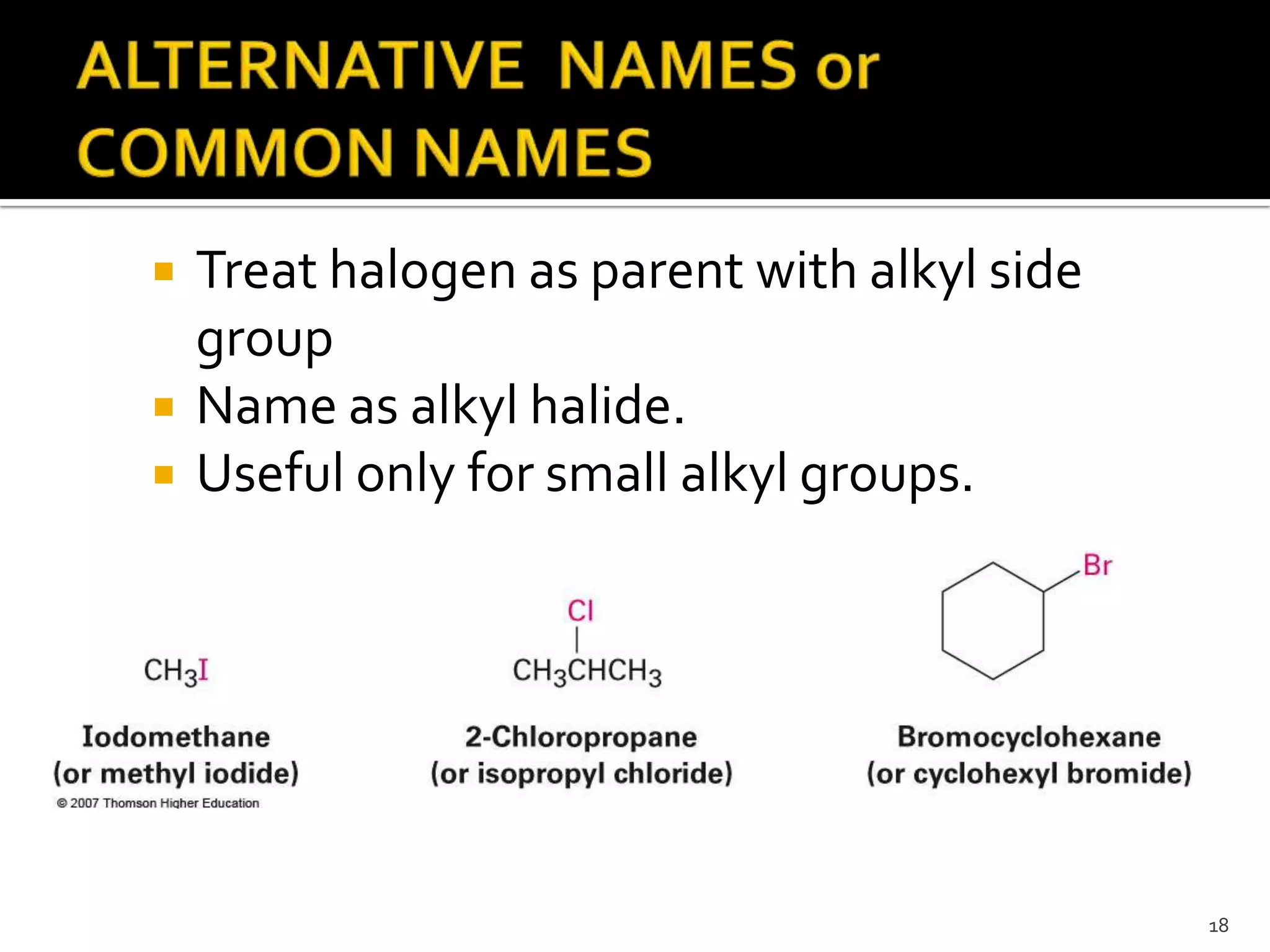
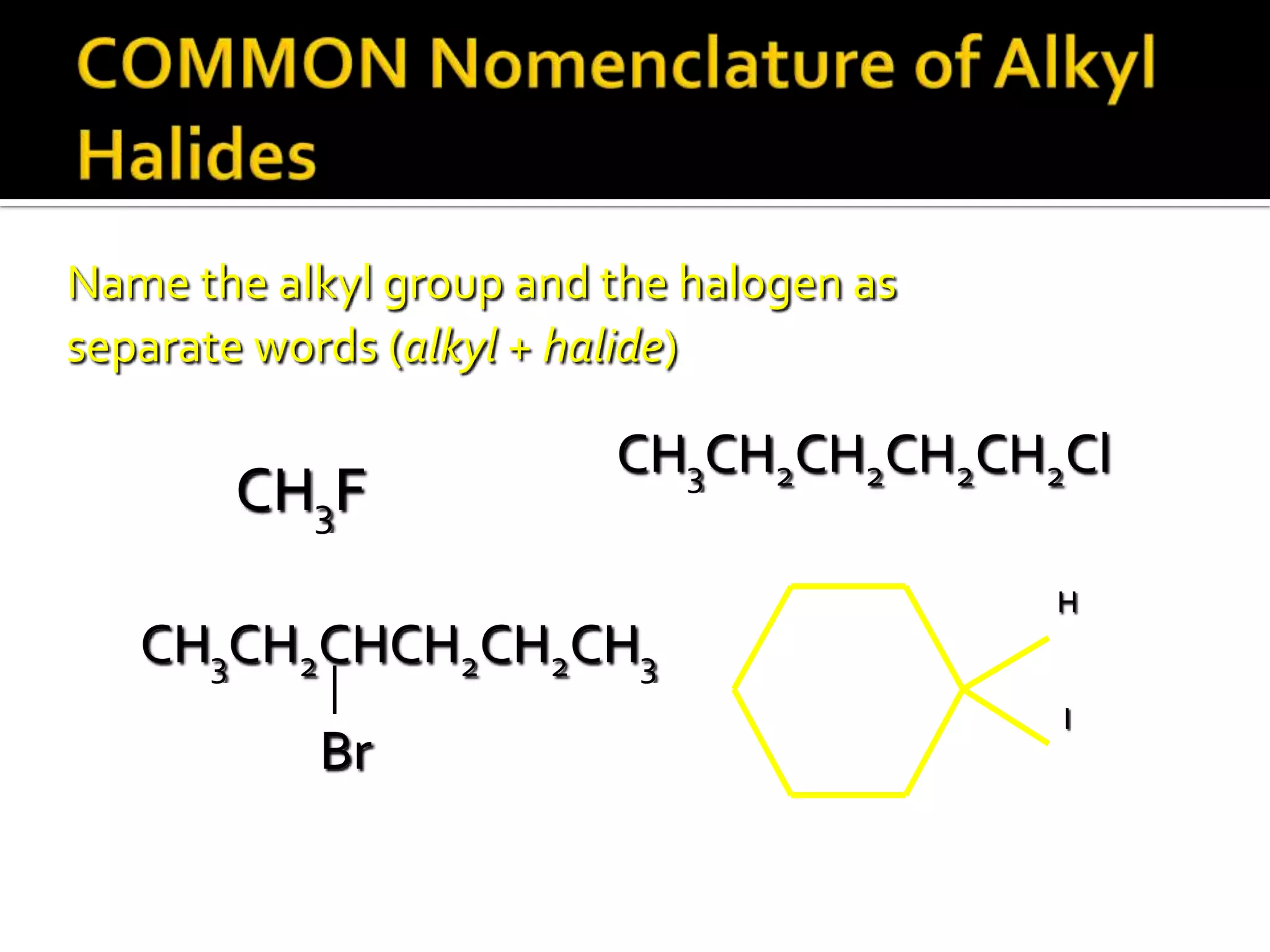
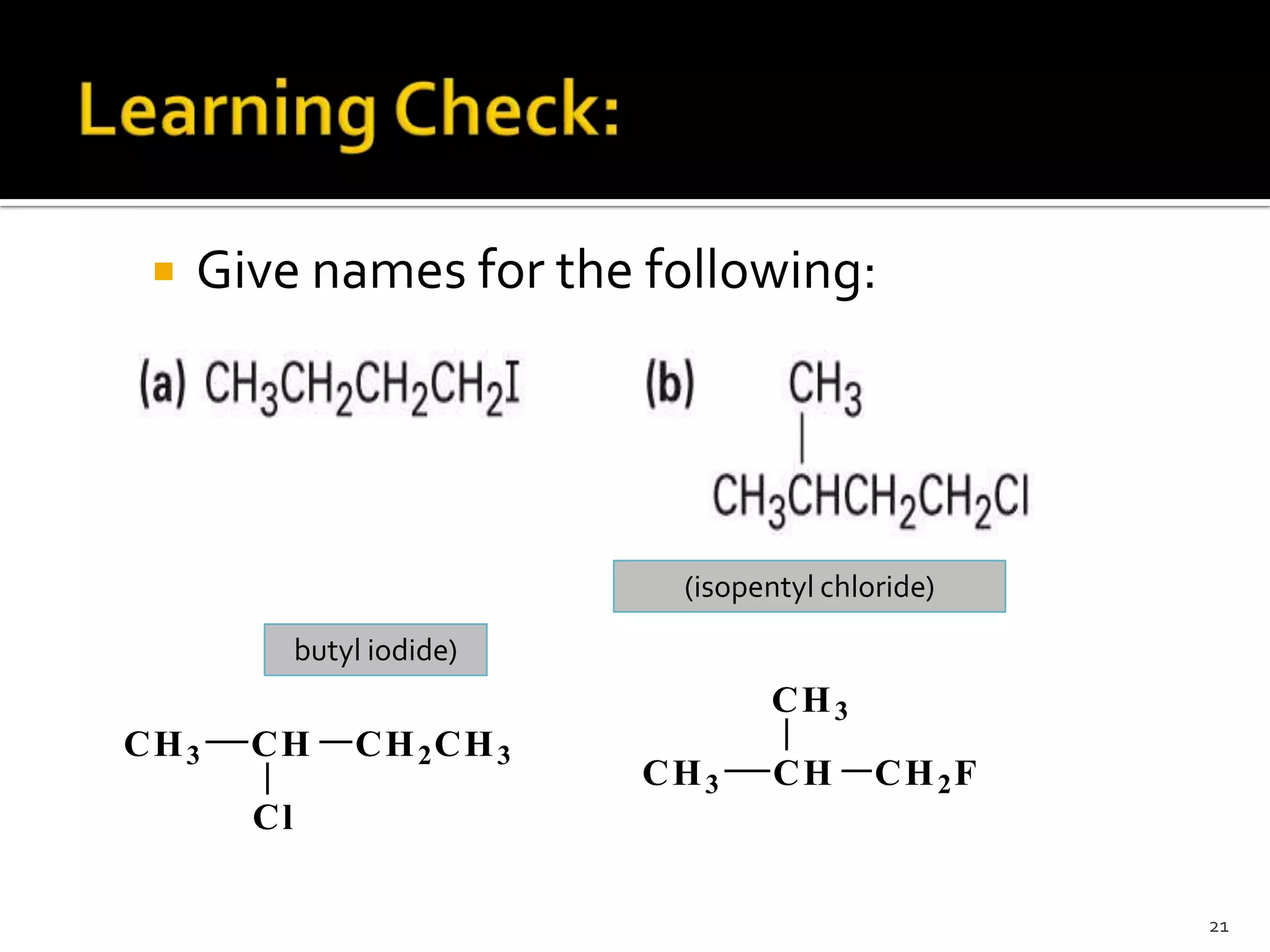
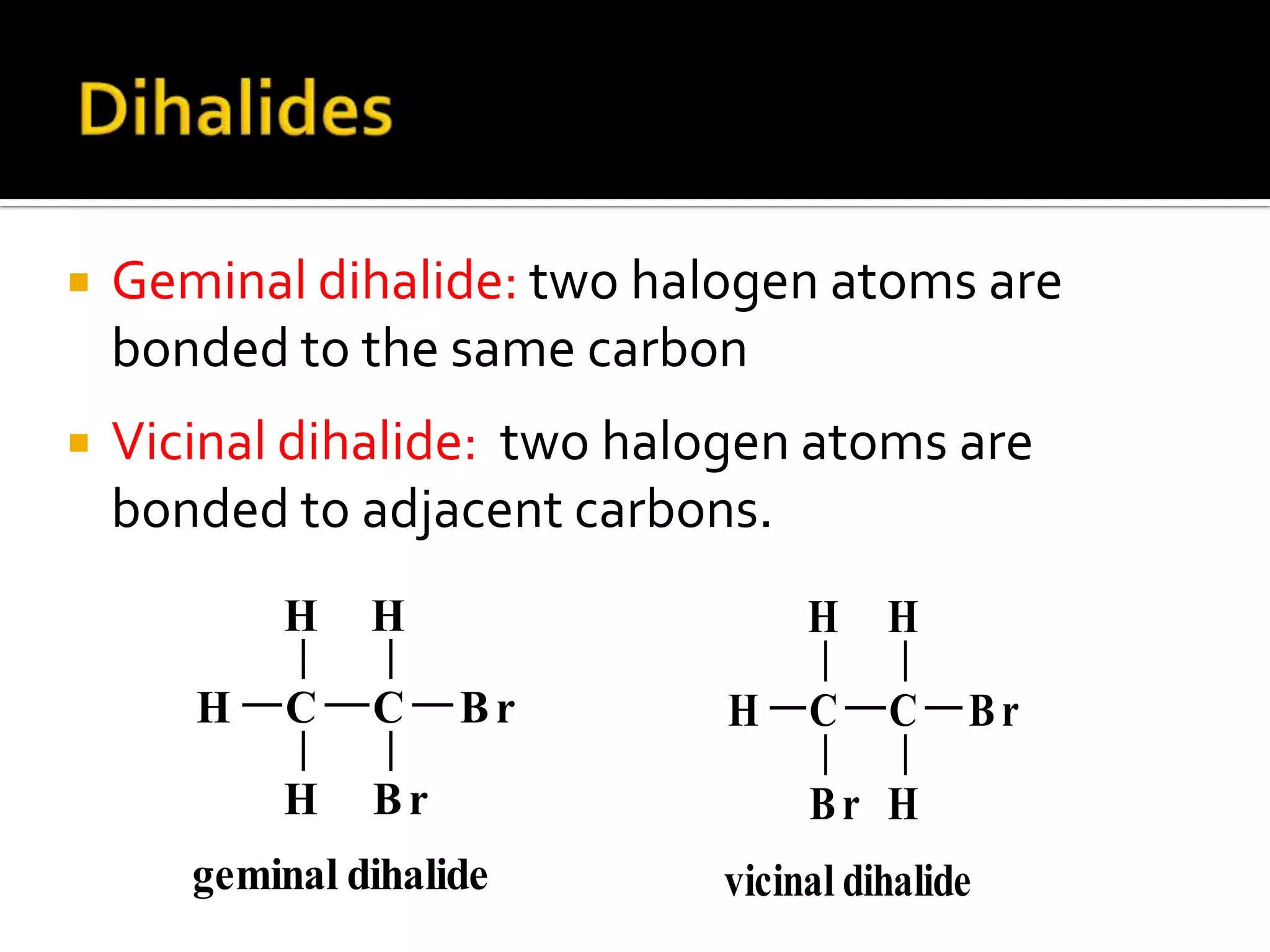
This document discusses alkyl halides, which are organic compounds containing carbon-halogen bonds. It classifies alkyl halides as primary, secondary, or tertiary depending on the carbon atom to which the halogen is bonded. The document explains how to name alkyl halides using IUPAC or common names based on parent chains and substituents. Examples of common alkyl halides are given along with their uses as solvents, anesthetics, refrigerants, and pesticides.