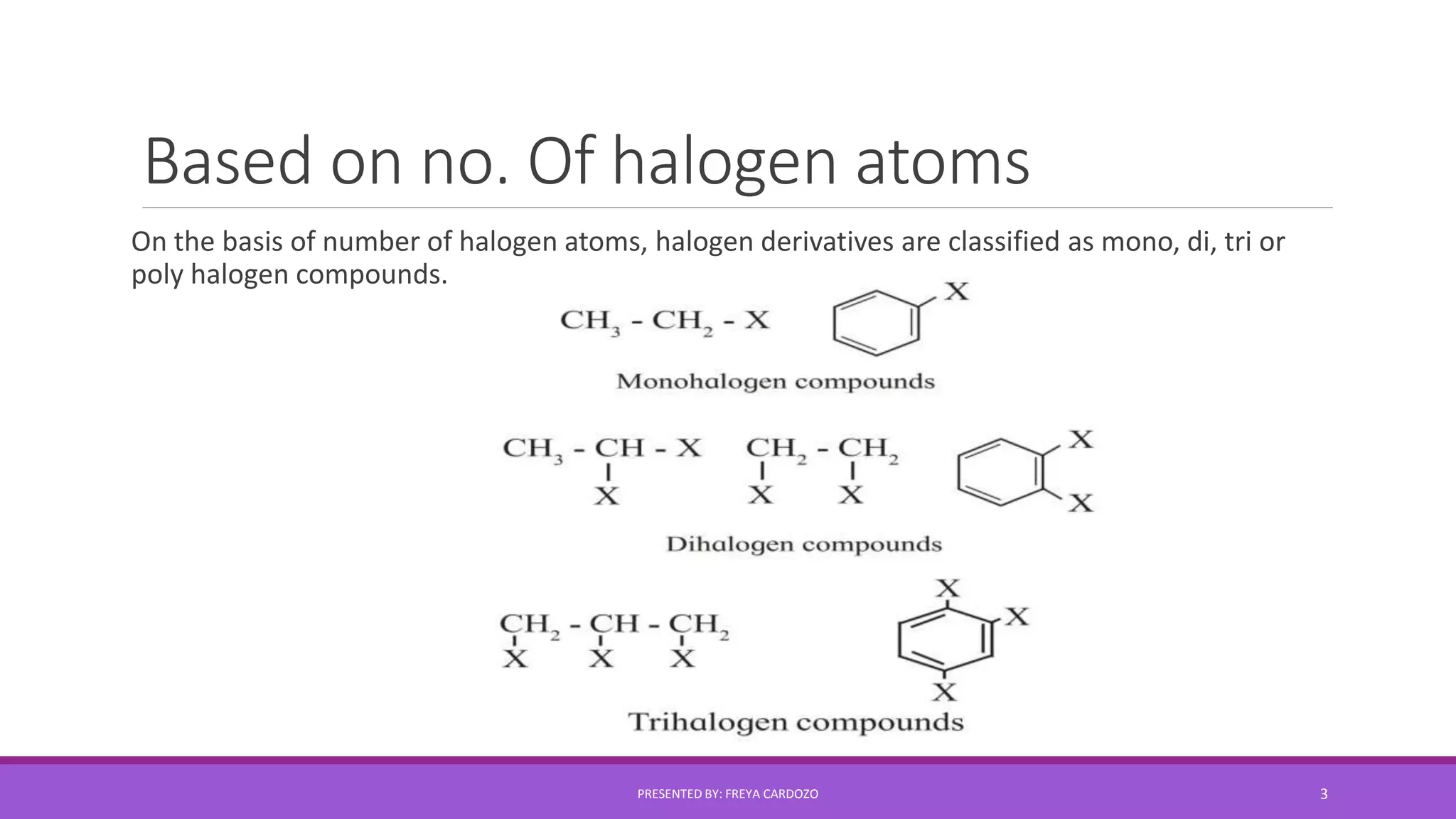
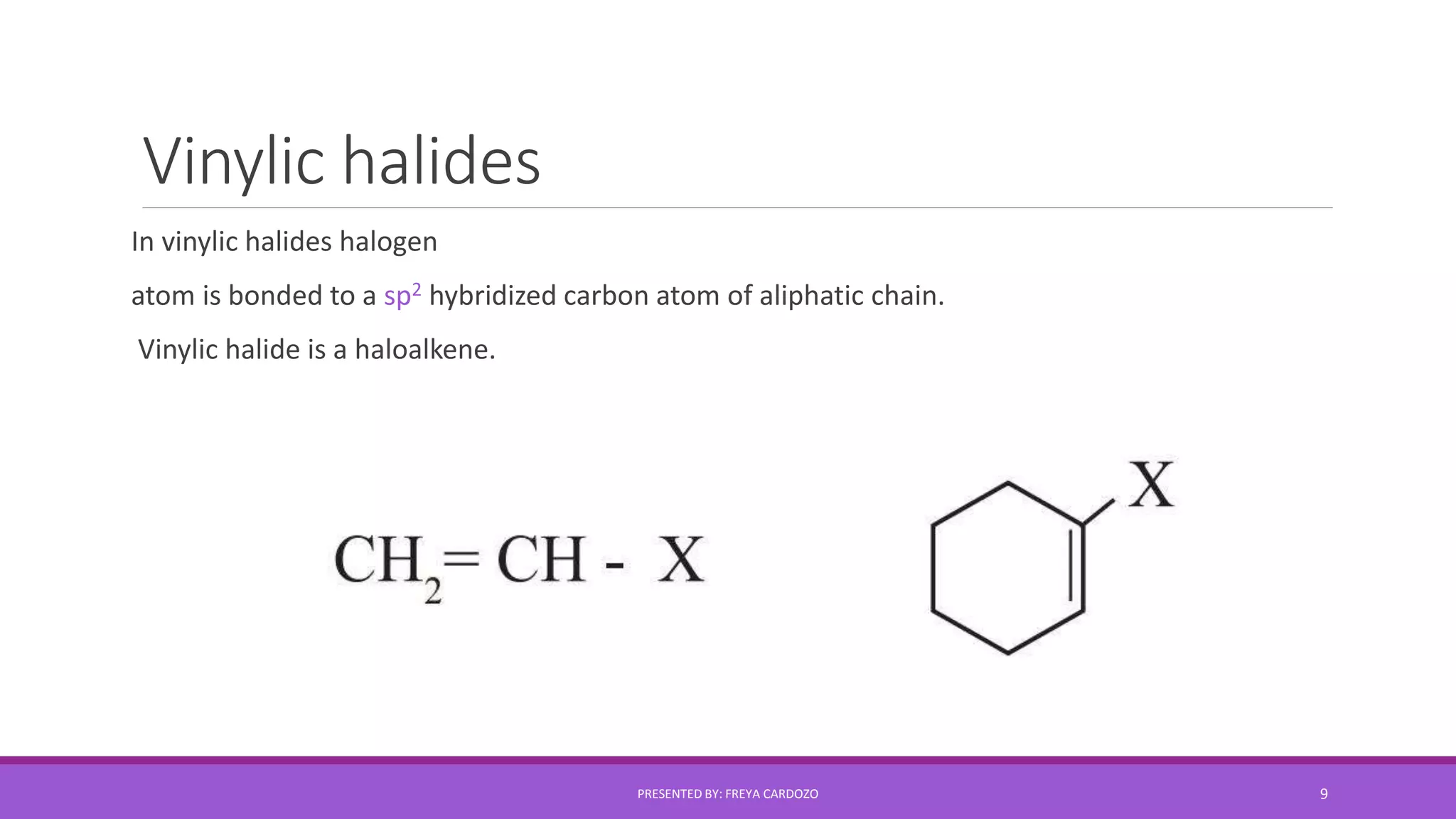
The document provides a comprehensive overview of halogen derivatives, classifying them based on the hydrocarbon skeleton and the number of halogen atoms. It discusses various types of alkyl halides, their preparation methods, physical properties, and reactivity, as well as optical activity and nucleophilic substitution mechanisms. Key chemical reactions and principles regarding solubility and boiling points of these compounds are also highlighted.